* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry Of The Human Body
Chemical reaction wikipedia , lookup
History of molecular biology wikipedia , lookup
Protein adsorption wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Citric acid cycle wikipedia , lookup
Click chemistry wikipedia , lookup
Water splitting wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Atomic theory wikipedia , lookup
Electrolysis of water wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Molecular dynamics wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Light-dependent reactions wikipedia , lookup
History of molecular theory wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Computational chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Transition state theory wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
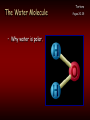
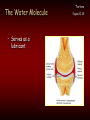
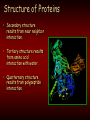
Chemistry Of The Human Body Structure & Function of Enzymes Introduction To Basic Chemistry Tortora Pages 20-25 • Structure of atoms. • Atomic number Vs atomic mass. • Role of electron shells Octet Rule; rule of eight. Introduction To Basic Chemistry Tortora Pages 20-25 • Covalent bonds – Sharing electrons – Outer shell electrons only – Rule of eight Molecule. • Ionic bonds – Transfer of outer shell electrons. Crystal. Introduction To Basic Chemistry Tortora Pages 20-25 • NOCH – Four most common occurring elements in the human body. – Why these four? • All can be in gaseous form. • All dissolve into water. • All form easily broken covalent bonds. • CHNOPS – Most common 6 elements. Introduction To Basic Chemistry • The most common 13 elements in the human body. (Exibit 2.1 Pages 21) • CHOPKINS café (is) Mg (if you add) Salt. Tortora Pages 20-25 Introduction To Basic Chemistry • Hydrogen bonds form between oxygen (-) and hydrogen (+) • Example: Water Tortora Pages 20-25 Introduction To Basic Chemistry • Chemical reactions • Dehydration synthesis – Making new molecules by extracting water. • Example: Linking amino acids to make a protein. Tortora Pages 20-25 Introduction To Basic Chemistry • Decomposition by adding water – Hydrolysis • Example: Digesting starch into glucose. Tortora Pages 20-25 Introduction To Basic Chemistry • Chemical reactions are either: • Endergonic: need more energy than they release. or Tortora Pages 20-25 Introduction To Basic Chemistry Exergonic: • Release more energy than they need. Tortora Pages 20-25 The Water Molecule • Why water is polar. Tortora Pages 20-25 The Water Molecule • Because water is polar: – Acts as a solvent. Tortora Pages 20-25 The Water Molecule • Participates in chemical reactions. Tuesday 9/10 Pages 20-25 The Water Molecule • Water absorbs and releases heat slowly. Tortora Pages 20-25 The Water Molecule • Requires a large amount of heat to change states. Tortora Pages 20-25 The Water Molecule • Serves as a lubricant. Tortora Pages 20-25 Fig 2-10 Homework Quiz • 1. T or F Triglycerides are the most common type of fat in the human body? • 2. T or F Carbon, oxygen, and nitrogen are the only three types of atoms found in fats? • 3. T or F Glycerol forms the three long chains found in a fat molecule? • 4. T or F Carbon atom forms the backbone of the fat molecule? • 5. T or F Fat molecules are saturated if they contain all double covalent bonds. • 6. T or F Fat molecules that are unsaturated have all straight fatty acid chains. • 7. T or F The fatty acid molecules are connected to a backbone glycerol molecule in a lipid molecules. • 8. T or F Double bonds in fats cause the fatty acids to bend and so are not straight. • 9. T or F Monounsaturated fats have 1 double bond. • 10. T or F Glycerol has three carbon atoms. ANSWERS 1. T 2. F 3. F 4. T 5. F 6. F 7. T 8. T 9. T 10. T Structure of Proteins • One of four classes of macromolecules. • Functions of proteins. • A polymer composed of many monomers (amino acids) • Peptides are short or incomplete proteins. Structure of Proteins • Typical amino acid. – – – – – Carbon Carboxyl group Hydrogen Amine group Radical • Amino acids are linked by dehydration synthesis. • Peptide bond is formed by water molecule being extracted. Structure of Proteins • Proteins are formed of polypeptide chains. • Proteins have four levels of structure. • Primary structure is determined by amino acid sequence and length. Structure of Proteins • Secondary structure results from near neighbor interaction. • Tertiary structure results from amino acid interaction with water. • Quarternary structure results from polypeptide interaction. Structure of Enzymes • Free energy is energy within a cell to do work. • A reaction that produces more free energy than it uses is exergonic. • A reaction the uses more free energy is endergonic. Activation Energy • Necessary input of energy for reaction to proceed. • Catalysis is the stressing of chemical bonds so as to require less activation energy. • Organic catalysts are enzymes. How Enzymes Speed Up Reactions • Speed up reactions by – Increase the frequency of collisions. – Position substrate molecules. Tortora P. 37 How Enzymes Speed Up Reactions • Speed up reactions by – Increase the frequency of collisions. – Position substrate molecules. Tortora P. 37 ATP: Energy Currency • Metabolism refers to sum of all energy use in the cell or body. – Energy use is necessary to maintain organization. – Energy is also needed for growth and reproduction. Tortora Page 33 ATP: Energy Currency • ATP acts as the cell’s battery. – Capable of storing energy. – Capable of releasing energy. Tortora Page 33 ATP: Energy Currency Tortora Page 33 • Structure of the ATP molecule. – 1 Adenine molecule + 1 ribose = adenosine. – 3 phosphates attached in a string. – High energy bonds between the phosphates. • Energy is stored as high energy covalent bonds between the phosphates. Each high energy bond equals 7.3 Kcal/mole. ATP: Energy Currency Tortora Page 33 • ATP and the release of energy. • ATP ADP + Pi + free energy. • ATP and the storage of energy • ADP + Pi + Free energy ATP ATP: Energy Currency • ATP - ADP Cycle Tortora Page 33 SCIENTIFIC METHOD 1. Starts with an observation and/or question? 2. Develop a hypothesis that’s predicts an outcome based on known information. 3. Design an experiment that will directly test the hypothesis. 4. Based on experimental evidence either support of reject the hypothesis. Developing a Hypothesis 1. Use the “If”, “Then” format. 2. If (a certain condition exists), Then (predict the results) 1) 2) 3) If substrate concentration is increased, Then reaction rate will increase. If enzyme concentration is increased, Then reaction rate will increase. If temperature is increased, Then reaction rate will increase.