* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Sulfuric acid wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Butyric acid wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
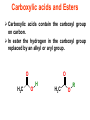
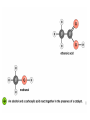
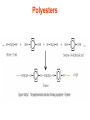
Esters • When alcohols are heated with carboxylic acids in the presence of concentrated sulfuric acid, they produce sweet smelling compounds called esters. • Because of their aroma and taste, esters are often incorporated into artificial perfumes and flavours. • They are also used as solvents and plasticisers. Carboxylic acids and Esters Carboxylic acids contain the carboxyl group on carbon. In ester the hydrogen in the carboxyl group replaced by an alkyl or aryl group. O H3C O O H H3C O R Esters Esters are sweet smelling organic compounds. Esters give their pleasant fragrances to fruits and flavors. Ethyl formate Raspberries contain ethyl formate; HCOOCH2CH3 You can make this compound from formic acid and ethanol. Ethyl butyrate Pineapples contain ethyl butyrate; CH3CH2CH2 COOCH2CH3 You can make this compound from butric acid and ethanol. Propyl acetate Pears contain propyl acetate; CH3COOCH2CH2CH3 You can make this compound from acetic acid and propanol. Esterification Reactions A simple reaction of this type is that of ethanol with ethanoic acid to form ethyl ethanoate: Intermolecular Esterification Formed from two different compounds by removing a mole of water in presence of an acid catalyst. It is also called Fisher Esterification (a classical transformation). Condensation process. It is a reversible reaction. Esterification H+ (catalyst) O R R OH R R OH Carboxylic Acid O Alcohol (a reversible condenstation reaction) O Ester H2O Esterification Reactions • In these reactions, known as esterification reactions, the small amount of sulfuric acid has two functions. • Firstly,and most importantly, the hydrogen ions act as a catalyst to increase the rate of the reaction and • Secondly it reacts with the water formed to shift the position of the equilibriumm to the right hand side (Le Chatelier’s principle) ensuring a good yield of product. • Note that unlike the acid and alcohol, an ester does not contain an –OH group and so is much more limited in its ability to hydrogen bond to water molecules, hence esters tend to be insoluble in water. Naming of Esters • It can be seen that the naming of esters is rather different named as from that of other organic compounds. • They are if they were salts of the alcohol and the acid; the alcohol provides the first half of the name (alkyl) and the organic acid provides the second half of the name (alkanoate). • In naming an ester it is important to remember that the – CO–group is part of the carboxylic acid. • The molecule below is therefore methyl propanoate (not propyl methanoate),because it can be considered as being formed from methanol and propanoic acid. Amides • Ammonia and primary amines initially react with carboxylic acids to form a salt of the acid, but if this is heated it dehydrates to form an amide. • If ethanoic acid is reacted with methylamine, and the initially formed methylammonium ethanoate is heated, then N-methylethanamide is formed: Polyesters • The two reactions discussed above are made use of in polymers known as condensation polymers. • In these polymers, two different functional groups are required and for each new bond between the monomer units (shown coloured below), a small molecule (often water) is produced. • Each monomer must also have two functional groups. • This can involve two different functional groups on the same monomer or more frequently, as in the examples below, two different monomers which have two identical groups on them. • One group of condensation polymers are the polyesters, so called because the bonding depends on the reaction of an alcohol with a carboxylic acid to form an ester. • The best known example of this polymer is Terylene, formed by the reaction of benzene–1.4–dicarboxylic acid with ethane–1.2–diol. as shown in Figure 1042(a) • The repeating unit in Terylene is therefore Polyesters