* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Meteorology Part 1
Precipitation wikipedia , lookup
Lockheed WC-130 wikipedia , lookup
Carbon dioxide in Earth's atmosphere wikipedia , lookup
Adiabatic process wikipedia , lookup
History of climate change science wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Automated airport weather station wikipedia , lookup
Hyperthermia wikipedia , lookup
Atmospheric circulation wikipedia , lookup
Water vapor wikipedia , lookup
Satellite temperature measurements wikipedia , lookup
Weather lore wikipedia , lookup
Tectonic–climatic interaction wikipedia , lookup
Surface weather analysis wikipedia , lookup
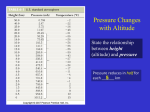
Warm Up 1. At 250 C, air contains 15 gH2O / m3 air. Saturation point: 20 g/m3 Calculate the relative humidity. 2. What is the dry adiabatic rate? 3. How does the temperature change within the thermosphere? WARM UP 1. What is the most abundant gas in the atmosphere? 2. What causes different layers in the atmosphere to form? 3. What is used to measure atmospheric pressure? 4. Where is the ozone layer located? WEATHER STATION MODELS The Weather Station Model ©Steve Kluge 2007 Some images from the NYSED Earth Science Reference Tables Decoding the Coded Pressure 196 Insert a decimal point to the left of the last digit 19.6 Put a “9” and a “10” in front of the result 1019.6 919.6 Test the results against the range of normal atmospheric pressures 919.6 is below the range of normal pressures…. REJECTED! 1019.6 is within the range of normal pressures…. ACCEPTED! Coded pressure 196= 1019.6 Decode these coded pressures: 002 1000.2 Back to the Station Model mb 993 280 000 999.3 1028.0 1000.0 Encoding the Pressure 1013.5 10135 Remove the decimal point Report the last 3 digits 135 Pressure 1013.5 = 135 encoded pressure Encode these pressures: 1032.7 327 Back to the Station Model 987.3 1012.2 873 122 1000.1 001 Back to Decoding Pressures The Barometric Trend + 19 / + means “Higher now than it was 3 hours ago” - means “Lower now than it was 3 hours ago” 19 means the pressure has changed by 1.9 mb / \ means the pressure is currently rising means the pressure is currently falling What was the pressure 3 hours ago? 1017.7 Here? 1003.5 Back to the Station Model ©S. Kluge 2007 NE at 25 Knots = 50 knots Back to the Station Model E at 5 Knots SE at 10 Knots Draw a Station Model for These Conditions: Temperature= 45F Dewpoint= 32F Wind NE at 20 knots Overcast Visibility= 1.5 miles Rain Showers Pressure Now= 997.3 mb Pressure 3 hrs. ago= 1000.2mb Barometer Falling Precipitation in last 6 hrs.= .53 in. ©S. Kluge 2007 Chapters 17 and 18 METEOROLOGY PART 1 Chapter 17: Atmosphere ATMOSPHERE - layer of gases and tiny particles surrounding the earth WEATHER - general atmospheric conditions at a particular time and place CLIMATE - general weather conditions over many years Composition of the Atmosphere Elements: NITROGEN (N2) OXYGEN (O2) ARGON (Ar) Compounds: CARBON DIOXIDE (CO2) WATER (H2O) OZONE (O3) absorbs harmful UV RAYS (ultraviolet) Composition of the Atmosphere ARGON Carbon Dioxide Atmospheric Dust: SOIL Ca ASH MICROBES CRYSTALS Nitrogen All Others Oxygen Atmospheric Pressure Gravity that is PULLING particles TOWARD EARTH Ratio of: air weight . surface area on which it presses Measuring Device for Atmospheric Pressure: BAROMETER (p.532) Atm.Pressure measured in N/m2. 1 Atm = 760 mmHg Atmospheric Pressure Δ Pressure: Higher altitude = FEWER gases = LOWER pressure Lower altitude = MORE gases = HIGHER pressure Δ Temperature: Higher altitude = LOWER pressure = LOWER temperature Lower altitude = HIGHER pressure = HIGHER temperature Atmospheric Layers Atmospheric Layering is caused by TEMPERATURE differences. AURORA Atmospheric Layers (1) TROPOSPHERE Closest to earth Holds the most CO2 and H2O vapor All WEATHER changes happen here Temperature ↓ as altitude increases. Why? FARTHER FROM THE HEAT ABSORBED BY EARTH 2) STRATOSPHERE From tropopause to 50km in altitude Includes the OZONE LAYER (O3) Temperature ↑ as altitude increases. Why? CLOSER TO O3 LAYER WHICH ABSORBS UV LIGHT & HEAT 3) MESOSPHERE From stratopause to 80km in altitude Coldest layer Temperature ↓ as altitude increases. Why? FARTHER FROM O3 LAYER (4) THERMOSPHERE From mesopause to outer space Temperature ↑as altitude increases. Why? OXYGEN AND NITROGEN ABSORB SHORT-WAVE, HIGH-ENERGY SOLAR RADIATION Two layers: IONOSPHERE - lower layer. Holds electrically charged particles. EXOSPHERE - upper layer. Holds light gases (helium/hydrogen). No clear boundary between exosphere and space… Air gets thinner and thinner until you’re in outer space. Atmospheric Moisture 3 forms of water: ICE, LIQUID, or WATER VAPOR (most is in VAPOR form) Phase Changes: HEAT energy causes an INCREASE in molecular motion. *Motion causes molecular COLLISIONS and energy transfer. PHASE CHANGES Evaporation: molecules speed up and change from a LIQUID to WATER VAPOR Condensation: molecules slow down and change from a GAS to a LIQUID. Sublimation: SOLIDS change directly to a GAS. (Ex: DRY ICE) Deposition: GASSES change directly to a SOLID. (Ex: FROST) LAB/DEMO: SUBLIMATE ME! Humidity = AMOUNT OF WATER VAPOR IN AIR SATURATED = air contains all of the water vapor it can hold. WHAT HAPPENS WHEN AIR IS SATURATED? WARM air can hold more water vapor than COLD air. Measuring Devices: HYGROMETER or PSYCHROMETER Specific Humidity = ACTUAL amount of moisture in the air. (Grams H2O / kg air) Relative Humidity = percent mass of water vapor compared to mass water vapor at saturation. Ex: At 200 C, air contains 14.3g H2O / m3 air. Saturation point: 17.1 g/m3 Specific Humidity: 14.3 g/m3 Relative Humidity: 14.3 g/m3 = 84% Relative Humidity Dew Point = TEMPERATURE to which the air must be cooled to reach saturation. Depends on Relative Humidity. When temp. is below Dew Point: CONDENSATION (dew) or DEPOSITION (frost) occur Temperature Changes occur in 3 ways: CONDUCTION: Transfer of heat through matter by molecular activity. CONVECTION: transfer of heat by mass movement or circulation within a substance. RADIATION: transfer of heat through matter or a vacuum by electromagnetic waves. Section 18.2 CLOUDS & FOG Cloud Formation = from CONDENSATION of water vapor over a large area of air. Land/Sea Breezes Why does Winnipeg’s temperature vary so much more than Vancouver’s? Vancouver is near the large ocean, which heats/cools slower than land. It holds that heat easily, keeping Vancouver’s air from fluctuating significantly. How do clouds affect Earth’s temperature during the day? Why? Clouds reflect light away from the ground, keeping the temperature lower. How do clouds affect Earth’s temperature during the night? Why? Clouds insulate the air, keeping heat from escaping, keeping the temperature higher. Types of Precipitation ***The type of precipitation that reaches Earth’s surface is determined by the temperatures in the lowest few kilometers of the atmosphere. Rain & Snow Sleet = small particles of clear-to-translucent ice. Glaze = A.K.A. “FREEZING RAIN” – rain is supercooled (below 0°C) & become ice when they impact frozen objects. Hail = small ice pellets grow as they impact supercooled water droplets as they fall through a cloud. UPDRAFTS push them back up, so they can gain new ice layers. ISOTHERM ACTIVITY ISOTHERMS & ISOBARS PRESSURE CENTERS & WIND ~section 19.2~ - Most common features on any weather map & weather generalizations can be made using them - Winds are influenced by pressure (pressure gradients) centers and the Coriolis effect ISOBARS = lines that connect points of equal air pressure What goes in, must come out!!!! When there is a converging air mass at the surface, it must be balanced by outflow - a surface CONVERGENCE can be maintained if a DIVERGENCE occurs above the low at the same rate as the inflow below and vice versa. Air spreads out (diverges) above surface cyclones and comes together (converges) above surface anticyclones