* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ultrastructural immunocytochemical localization of
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Tissue engineering wikipedia , lookup
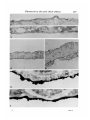
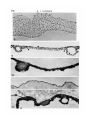
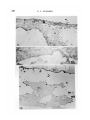
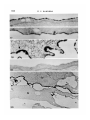
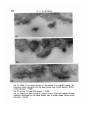
/ . Embryol. exp. Morph. Vol. 71, pp. 155-170, 1982 Printed in Great Britain © Company of Biologists Limited 1982 \ 55 Ultrastructural immunocytochemical localization of fibronectin in the early chick embryo ByE. J. SANDERS1 From the Department of Physiology, University of Alberta SUMMARY Fibronectin was localized using peroxidase and biotin-avidin-ferritin techniques in developmental stages of the chick embryo from laying to primitive streak formation. The primary location of fibronectin at all stages examined was the basal lamina of the epiblast and its associated extracellular material. The remaining tissues showed little or no immunochemical deposit. At the primitive streak stage of development there were some regional differences in the density of the deposit on the basal lamina. Closely adjacent to the primitive streak, where the basal lamina is fragmentary, deposit was sparse or absent. In this, and other regions, mesoderm cell surfaces did not stain except where they closely approached the stained basal lamina or interstitial bodies. Staining was variable in the basal lamina anterior to the primitive streak, but in a number of cases particularly heavy deposit was noted overlying the crescent of late hypoblast. This area seemed to correspond with the anterior fibronectin-rich band reported by others using immunofluorescence localization. INTRODUCTION The morphogenetic movements of gastrulation involve the coordinated and directed movements of several early embryonic cell populations (Vakaet, 1970; Nicolet, 1971). It is currently widely held that extracellular materials, present in the immediate vicinity of these migrating cells, exert some controlling influence over the extent and direction of these movements. One of the most strongly implicated substances is fibronectin which, in a number of different developmental situations, is ideally positioned both spatially and temporally to modulate morphogenetic migration (Wartiovaara, Stenman & Vaheri, 1976; Wartiovaara et al., 1978; Wartiovaara, Leivo & Vaheri, 1979; Spiegel, Burger & Spiegel, 1980; Armstrong & Armstrong, 1981; Lesot, Osman & Ruch, 1981; Silver, Foidart & Pratt, 1981; Thesleff et al., 1981). In the chick embryo, fibronectin has been identified in a number of significant locations at developmental stages subsequent to gastrulation (Linder, Vaheri, Ruoslahti & Wartiovaara, 1975; Newgreen & Thiery, 1980; Waterman & Balian, 1980; Mayer, Hay & Hynes, 1981). Immunofluorescent technique on whole mounts also indicates the presence of fibronectin in chick embryos at 1 Author's address: Department of Physiology, University of Alberta, Edmonton, Alberta, Canada T6G 2H7. 156 E. J. SANDERS Fig. 1. Diagrammatic representation of the stage-5 chick embryo showing the regressing primitive streak and the anterior crescent of 'late hypoblast'. The figures refer to the levels at which these embryos were routinely sectioned. gastrulation stages, principally associated with the basal lamina of the epiblast and most particularly in a band of fibres in the anterior of the area pellucida (Critchley, England, Wakely & Hynes, 1979; Wakely & England, 1979), where it may influence the spreading and migration of cells. Furthermore, in vitro studies have clearly shown that the presence of fibronectin on the substratum markedly increases the rate of spreading of mesoderm taken from primitivestreak-stage embryos (Sanders, 1980). This enhancement was observed both on substrata pretreated with plasma fibronectin and on substratum-attached material derived from early embryonic epithelia (endoblast and hypoblast). In order to further study the possible role of fibronectin in gastrulation, and the migration of mesoderm cells in particular, we have sought to localize this substance in the early embryo more precisely. Ultrastructural techniques were used on developmental stages from laying to gastrulation. The techniques used were the peroxidase-antiperoxidase (PAP) method (Sternberger, 1979) and Fig. 2. Transmission electron micrograph (TEM) showing the dorsal surface of the epiblast of a stage-5 embryo incubated under PAP control conditions. There is some membrane intensification, x 37100. Fig. 3. TEM showing the basal lamina on the ventral surface of the epiblast of a stage-XIII embryo incubated under PAP control conditions, x 37100. Fig. 4. Light micrograph (LM) of a section through a PAP-incubated stage XI embryo. The basal lamina on the ventral surface of the epiblast is stained, but the dorsal surface of the epiblast is not. x 480. Fig. 5. TEM of the basal lamina of a stage-XI embryo. PAP technique, x 37100. Fig. 6. LM of a section through a PAP-incubated stage-XIII embryo. The basal lamina stains but the other surfaces of the epiblast and hypoblast do not. x 480. Fig. 7. TEM of the basal lamina of a stage-XIII embryo. PAP technique, x 37100. Fibronectin in the early chick embryo 157 EMB 71 158 10 12 E. J. SANDERS Fibronedin in the early chick embryo 159 biotin-avidin-ferritin (BA) method (Heitzmann & Richards, 1974; Skutelsky, Danon, Wilchek & Bayer, 1977). The results show that fibronectin is present in the embryo from the time of laying, mainly in the epiblast basal lamina and the associated interstitial bodies. Some regions of the area pellucida showed more intense immunolabelling than others and the basal lamina appeared devoid of fibronectin adjacent to the primitive streak. MATERIAL AND METHODS Embryos were used at three different stages of development: (i) unincubated, at stage X or XI of Eyal-Giladi & Kochav (1976); (ii) 6 h incubation, at approximately stage XIII; and (iii) 24 h incubation at stage 5 of Hamburger & Hamilton (1951). The embryos were dissected free of the vitelline membrane and yolk in Pannett & Compton's saline and fixed with 0-5% glutaraldehyde in 0-1 M cacodylate buffer, pH 7-4, for 10 min at room temperature. Trials with fixative concentrations from 0-1 % to 2-5% showed that 0-5% was most satisfactory in terms of retaining acceptable morphology and producing the least non-specific labelling. After this brief fixation, the embryos were washed overnight in vials on a rotating mixer with three changes of phosphate-buffered saline (PBS) containing 1% normal goat serum (g.s.) and 0-1 M lysine. The goat serum, present at several stages of the preparation, was used to help prevent subsequent non-specific immunolabelling (Sternberger, 1979), and the lysine was used to quench the glutaraldehyde and available aldehyde groups. In some experiments, the goat serum was replaced by 1 % bovine serum albumin. This substitution made no difference to the quality of the control samples. For the PAP method, embryos were incubated in rabbit anti-human fibronectin antiserum (Collaborative Research Inc.), diluted 1:100 with PBS + g.s., for 2 h. All incubations and washes were carried out at room temperature on a Fig. 8. LM through the primitive streak of a stage-5 embryo. PAP technique. The basal lamina is the most densely stained structure, and this terminates in the region of mesoderm invagination x 125. Fig. 9. LM of a conventionally stained section through the anterior region of the area pellucida of a stage-5 embryo. The section is taken at approximately level 1 indicated in Fig. 1. The arrowheads mark the boundary of the centrally located endoblast and the peripherally located late hypoblast. The latter is thrown into characteristic folds, x 40. Fig. 10. TEM of the basal lamina of a stage-5 embryo. Note the large interstitial body which stains heavily at its periphery. PAP technique, x 37100. Fig. 11. TEM of the dorsal surface of the epiblast of a stage-5 embryo. PAP technique x 37100. Fig. 12. TEM of the stage-5 basal lamina overlying the anterior crescent of late hypoblast. PAP technique, x 37100. 6-2 160 E. J. SANDERS •*.<«* * , * » « : • • ^ ,~ En /. 4 '1 15 * * * Fibronectin in the early chick embryo 161 rotating mixer. This was followed by an overnight wash with five changes of PBS + g.s. The specimens were then incubated for 1 h in goat anti-rabbit IgG antiserum (Miles Laboratories Inc.), diluted 1:10 in PBS + g.s. After rinsing for 1 h with two changes of PBS + g.s., the embryos were incubated in PAP reagent (Miles), diluted 1:50 with PBS + g.s. for 30 mins and then rinsed twice with PBS alone for 10 min. Specimens were then immersed in diaminobenzidine (DAB) reagent for 20 min and then washed with PBS for 30 min with three changes. The DAB reagent was freshly made in clean glassware by adding 0-01 % hydrogen peroxide to 0-05% DAB in PBS. The material was postfixed with 1 % osmium tetroxide in 0-1 M phosphate buffer, pH 7-4, for 1 h, dehydrated in ethanol and propylene oxide and embedded in Araldite. Three different controls were run: (i) fibronectin antiserum replaced by normal rabbit serum; (ii) fibronectin antiserum replaced by PBS; and (iii) all steps up to the DAB reagent replaced with PBS + g.s., with a wash in PBS alone immediately before the DAB treatment. Penetration of the PAP reagents into the tissue spaces did not appear to present any problem, and reaction product was frequently seen on tissue surfaces that were closely apposed. For the biotin-avidin-ferritin method, embryos were fixed and washed as before. With stage-5 embryos the endoblast was dissected off with tungsten needles either before or after fixation, in order to facilitate penetration of the avidin-ferritin to the basal lamina. Incubation with antifibronectin antiserum and the subsequent wash was performed as in the PAP method. Embryos were then incubated in biotinylated antirabbit IgG (Vector Laboratories Inc.), diluted 1:5 with PBS + g.s., for 1 h. After washing with PBS + g.s. for 1 h with two changes, the specimens were immersed in ferritin-congugated avidin D (Vector), diluted 1:10 with PBS 4-g.s., for 30 min. Embryos were then washed with PBS alone, postfixed with osmium tetroxide, dehydrated and embedded as described above. Three different controls were run: (i) fibronectin antiserum replaced by normal rabbit serum; (ii) fibronectin antiserum replaced by PBS; and (iii) fibronectin antiserum and biotinylated IgG replaced by PBS. This last mentioned control was used to check for endogenous avidin-binding activity (Wood &Warnke, 1981). Specimens were thin sectioned for electron microscopy without staining, and examined on copper grids with a Philips 300 microscope. Specimens at stages XI Fig. 13. LM of a stage-5 embryo through the late hypoblast crescent. PAP technique. The basal lamina is most heavily stained over the folded late hypoblast, (H). Ep. = epiblast. x 300. Fig. 14. TEM of the basal lamina in the primitive streak region of a stage-5 embryo. Note that the basal lamina is poorly organized and fails to stain. PAP technique, x37100. Fig. 15. TEM through the anterior region of a stage-5 embryo showing the epiblast (Ep) and endoblast (En). PAP technique, x 4460. 162 E. J. SANDERS f 20 Fibronectin in the early chick embryo 163 and XIII were sectioned in the centre of the area pellucida. Stage-5 embryos were examined in detail by sectioning in at least five different regions (see Results). For light microscopy, 1 /*m sections were examined either unstained (PAP method) or stained with methylene blue and azure B. RESULTS The unincubated embryo is characterized by a single epithelial layer, the epiblast, underlaid by an organized but incomplete basal lamina (Low, 1967; Sanders, 1973, 1979) and scattered hypoblast cells (Eyal-Giladi & Kochav, 1976). By 6 h incubation, both the basal lamina and hypoblast have been completed. The stage-5 embryo (Fig. 1) is characterized by a regressing primitive streak (Nicolet, 1971) and a mesoderm layer underlaid by endoblast which is displacing the hypoblast (Sanders, Bellairs & Portch, 1978). The four tissues which have been examined here are therefore: epiblast, hypoblast, endoblast and mesoderm. PAP Technique The control samples run with every experiment were largely negative for electron-dense deposit (Figs. 2 and 3). In some control samples the dorsal surface of the epiblast and the endoblast surfaces showed an intensification of membrane staining (Fig. 2) but was of a low level and was taken into consideration in interpreting results. The controls in which DAB was used without prior antibody incubation showed no deposit and therefore no random adsorption of DAB. (i) Stage XL At this stage the ventral surface of the epiblast stained for fibronectin while the dorsal surface did not (Fig. 4). Details of the basal lamina on the ventral surface were obscured by the dense and uneven layer of deposit (Fig. 5). (ii) Stage XIII. Despite the presence of a hypoblast layer in this stage, the only surface to react positively was the basal lamina of the epiblast (Fig. 6). As in stage XI details of the lamina were obliterated by the dense deposit (Fig. 7). Fig. 16. TEM of the dorsal surface of the endoblast. Stage-5 embryo. PAP technique, x37100. Fig. 17. TEM of the ventral surface of the endoblast. Stage-5 embryo. PAP technique, x 37100. Fig. 18. TEM of the surface of a late hypoblast cell of a stage-5 embryo. PAP technique. Note the association of the deposit with membrane invaginations. One invagination (arrowhead) appears to be a coated pit. x 55 600. Fig. 19. TEM of the surface of a mesoderm cell remote from the basal lamina. Stage-5 embryo. PAP technique, x 37100. Fig. 20. TEM of a stage-5 embryo, showing the epiblast (Ep) and its basal lamina with closely apposed mesoderm cell (M). PAP technique, x 11700. 164 E. J. SANDERS 22 23 Fig. 21. TEM of the ventral surface of the epiblast of a stage-XI embryo. The interstitial bodies associated with the basal lamina show ferritin deposits. Biotinavidin technique, x 55 600. Fig. 22. As Fig. 21, stage-XIII embryo, x 55600. Fig. 23. TEM of the basal lamina of a stage-5 embryo. The ferritin deposit is more uniformly distributed on the basal lamina than in earlier stages. Biotin-avidin technique, x 46100. Fibronectin in the early chick embryo 165 (iii) Stage 5. In view of the relative complexity of the stage-5 embryo, sections through at least five different regions were examined on each embryo, as shown in Fig. 1. Sections were made through the primitive streak, at various levels, including areas lateral to this structure (Fig. 8). In addition, sections were taken in the area pellucida anterior to Hensen's node, passing through the crescent of loosely attached ventral cells characterized by their foamy appearance, and sometimes known as the 'germinal crescent' (Nicolet, 1971). These cells are termed 'late hypoblast' here, Fig. 1, and are clearly distinguishable from the endoblast by both light and electron microscopy, by their increased yolk content and larger size (Fig. 9). (a) Epiblast and basal lamina. As illustrated in Fig. 8, the basal lamina was by far the most fibronectin-rich tissue surface in the embryo in all regions examined. Although there was some decrease in intensity from the level of Hensen's node to more posterior regions, all areas showed deposit both on the basal lamina proper and on the associated interstitial bodies (Fig. 10), and occasional extracellular filaments. This was in sharp contrast to the dorsal surface of the epiblast, which showed little or no deposit in comparison with controls (Fig. 11). Anterior to Hensen's node the situation was variable. However, in a number of cases the basal lamina overlying the late hypoblast in this region presented a much denser deposit than that overlying the endoblast (Figs 12 and 13). It is unlikely that the variability of this region was due to impaired accessibility of the reagents, since equally inaccessible regions showed ample deposit. As the basal lamina enters the region of the primitive streak or Hensen's node it first begins to fragment, and then disappears entirely in the zone of actively invaginating cells (Low, 1967). The PAP staining was of particular interest in this area. As expected, light microscopy showed a tapering off of the stained basal lamina as it approached the primitive streak, Fig. 8. Ultrastructural examination showed that nearest the streak or Hensen's node, in the fragmenting region, the lamina failed to stain (Fig. 14), and a transition could be observed between the stained and unstained areas of the basal lamina. (b) Lower layer (endoblast and late hypoblast). In comparison with the basal lamina, all other tissue surfaces showed minimal staining. The endoblast presented a somewhat patchy deposit (Fig. 15) but both the dorsal and ventral surfaces of this tissue largely possessed a thin, uniform layer of reaction product (Figs 16 and 17). The late hypoblast in the anterior of the area pellucida (see Fig. 1) showed a patchy staining with the deposit noticeably associated with apparent pinocytotic invaginations of the cell surface (Fig. 18). In some places the invaginations appeared to be coated pits (Fig. 18). This characteristic distribution was not seen in stage-XIII hypoblast or in any other tissue. (c) Mesoderm. These cells were consistent in their reaction, and showed little or no deposit within the bulk of the tissue (Fig. 19). Where mesoderm cells were closely apposed to the basal lamina (Fig. 20) or the dorsal surface of the endo- 166 E. J. SANDERS blast they were frequently associated with dense patches of material, possibly interstitial bodies. BA Technique The controls run for this method were always reliable and ferritin was routinely found to be absent from these embryos. In the experimental series of embryos at all stages, the only surface found labelled was the basal lamina of the epiblast (Figs. 21, 22, 23). Labelling was consistent between individual embryos but of relatively low density in comparison with PAP. For this reason regional differences in density across the area pellucida were difficult to discern. Other than the selective labelling of the ventral surface of the epiblast, the only generalization possible with certainty was that in the early stages (XI and XIII) and the interstitial bodies were primarily labelled (Figs. 21 and 22), while at stage 5 label was found both on these bodies and on the basal lamina proper (Fig. 23). DISCUSSION The finding that fibronectin was present in the basal lamina was not unexpected, since this has been repeatedly demonstrated in other tissues in recent work (Wartiovaara et al. 1976, 1978; Courtoy, Kanwar, Hynes & Farquhar, 1980; Madri, Roll, Furthmayr & Foidart, 1980; Waterman & Balian, 1980; Lesot et al. 1981; Repesh, Furcht & Smith, 1981; Silver et al. 1981; Thesleff et al 1981). The present results are of particular interest in view of the early stages in the development of the basal lamina used here. Fibronectin was found in this structure shortly after the time of laying with little difference in labelling intensity from that in the gastrulating embryo. Formation of the basal lamina during this developmental period has been followed in some detail (Low, 1967; Sanders, 1979) and the gradual morphological elaboration described. It would now be of interest to examine the basal lamina before the time of laying to determine the relationship between the formation of this structure and the appearance of fibronectin. Mitrani & Farberov (1981) report that no fibronectin is detectable by immunofluorescence at in utero stages of development. Interstitial bodies, found closely adjacent to the basal lamina, were originally described by Bellairs (1963) and Low (1970), and have recently been shown to contain fibronectin at later stages of development than those used here (Mayer et al. 1981). In the present case these bodies, like the basal lamina, are shown by the PAP method to contain fibronectin from stage XI onwards. Results with the less sensitive BA method would indicate less fibronectin in the basal lamina than the interstitial bodies at the earlier stages. It is possible that the interstitial bodies reorganize and contribute material, including fibronectin, to the developing basement membrane. From a morphogenetic point of view, therefore fibronectin is present in the tissue space, between the upper and lower layers, well in advance of the appearance of the migratory mesoderm cells. This situation is also the case with regard to the advance deposition of fibronectin the Fibronectin in the early chick embryo 167 migratory pathway of neural crest cells described by Newgreen & Thiery (1980). This led these authors to conclude that the appearance of fibronectin in itself was not sufficient to initiate cell migration, although the role of fibronectin in the subsequent facilitation of migration is a clear possibility. Label was not found associated with the mesodermal cell surfaces here except where these cells came into the vicinity of the basal lamina. At this stage of development extracellular filaments are rarely observed (Sanders, 1979). The mass of the mesoderm is several cell layers thick at stage 5 (Ebendal, 1976; England & Wakely, 1977) and many cells, which apparently have no contact with the basal lamina, are therefore minimally associated with fibronectin. Although the possibility exists that extracellular scaffolding materials, with or without fibronectin, have not been preserved or visualized by the current technique, it is difficult to envisage how fibronectin could be influencing cells in the bulk of the mesoderm. It is possible that the spreading of only those mesoderm cells actually in contact with the basal lamina and/or interstitial bodies is actively influenced by the presence of fibronectin, and these cells in turn influence the remaining cells deeper within the mesoderm tissue. Such a spread of influence has been suggested to operate in the interrelationship between basal lamina and limb bud mesenchymal cells (Lunt & Seegmiller, 1980). In this way the cells in immediate contact with the fibronectin-rich extracellular materials may be induced to spread and migrate away from the primitive streak and this movement of cells might stimulate mesoderm cells more remote from the fibronectin to behave similarly, perhaps by contact guidance. The basal lamina immediately adjacent to the zone of actual invagination in the primitive streak was found to be devoid of label. This correlates with the observation that cells newly invaginated tend to be rounded, while more laterally they tend to be more flattened or irregularly shaped (Solursh & Revel, 1978), although such flattening is in no way comparable with that observed in vitro (Sanders, 1980), where flattening is extreme. Wakely & England (1977) also report changes in mesoderm cell morphology as cells move away from the primitive streak. Such circumstantial correlations tend to strengthen the speculation that fibronectin has an in vivo role in control of cell shape in this situation. The factors responsible for the difference in fibronectin content of the basal lamina in the primitive streak region are unclear. In agreement with the present results, in vitro studies of these tissues (Sanders, 1980) have shown that the mesoderm at this stage lacks the ability to synthesize large quantities of fibronectin, and in addition, that these cells are very responsive to substratum-attached fibronectin. Also, in culture, stage-XIII hypoblast and stage-5 endoblast were shown to produce abundant cell surface fibronectin. As shown here in situ, however, these two tissues are associated with relatively little fibronectin and much less than is the basal-lamina-bearing epiblast. This difference may be a reflection of the response of the endoblast and hypoblast to the culture conditions. 168 E. J. SANDERS Observations here show that the late hypoblast bound label in apparent endocytic invaginations, some of which appeared to be coated pits. This may be compared with the extensive coated vesicle activity in yolk-sac endoderm (Mobbs & McMillan, 1981), since the latter tissue is thought to derive from hypoblast via extraembryonic endoderm (Fontaine & Le Douarin, 1977). In the yolk sac this endocytosis appears to be involved with yolk protein sequestration, but whether the hypoblast is active in this process is unclear. It is usually considered that the extraembryonic endoderm is the primary site of yolk uptake (Bellairs, 1963). Previous work on gastrulating embryos has localized fibronectin using whole-mount immunofluorescence technique (Critchley et al 1979; Wakely & England, 1979). One of the main findings from these studies was that fibronectin was particularly abundant in the basal lamina in a well-defined crescent, corresponding with the position of the 'germinal crescent' at the anterior of the embryo at the boundary of the area pellucida and area opaca. The present results show a general diminution of label on the basal lamina on passing from the anterior to the posterior of the embryo, but not a consistently appearing, well-defined, anterior band of intense label. This may be due to the high sensitivity of the PAP method, which results in heavy deposits across the entire basal lamina thus obscuring an anterior band and to the relatively low sensitivity of the BA method. However, in a number of embryos (Fig. 12) particularly heavy deposit was observed in the basal lamina overlying the anterior crescent of late hypoblast (germinal crescent). This tissue has been shown by several studies to form from the original hypoblast by cranial compression (Vakaet, 1962; Rosenquist, 1972; Fontaine & Le Douarin, 1977), and perhaps to give rise to primordial germ cells (Vakaet, 1962,1970). Since there is a developmental continuity between the stage-XI hypoblast and the stage-5 anterior crescent, and since the former shows the ability in culture to secrete large amounts of fibronectin, it seems possible that the hypoblast may contribute to the copious amount of fibronectin sometimes seen in the basal lamina of the anterior crescent. The variability observed in this area could possibly be due to imprecise staging of the embryos, although this would require that the fibronectin-rich crescent was itself precisely timed in its appearance. Therefore, apart from the variability, the speculations of Critchley et al. (1979) and Wakely & England (1979), regarding a possible role for fibronectin in germ cell migration from the anterior crescent, are not contradicted by the present observations. The reason why these cells would require a substratum much richer in fibronectin than does the mesoderm is not apparent, unless it is to provide the specific direction required by the germ cells as apposed to the general centrifugal direction of the mesoderm. I am indebted to Mrs Sita Prasad for careful technical assistance and to Dr S. E. Zalik for reading and criticizing the manuscript. This work was supported by a grant from the Medical Research Council of Canada. Fibroneclin in the early chick embryo 169 REFERENCES ARMSTRONG, P. B. & ARMSTRONG, M. T. (1981). Immunofluorescent histological studies of the role of fibronectin in the expression of the associative preferences of embryonic tissues. J.Cell Sci. 50,121-133. BELLAIRS, R. (1963). Differentiation of the yolk sac of the chick studied by electron microscopy. /. Embryol. exp. Morph. 11,201-225. COURTOY, P. J., KANWAR, Y. S., HYNES, R. O. & FARQUHAR, M. G. (1980). Fibronectin localization in the rat glomerulus. /. Cell Biol. 87,691-696. CRITCHLEY, D. R., ENGLAND, M. A., WAKELY, J. & HYNES, R. O. (1979). Distribution of fibronectin in the ectoderm of gastrulating chick embryos. Nature 280, 498-500. EBENDAL, T. (1976). Migratory mesoblast cells in the young chick embryo examined by scanning electron microscopy. Zoon, 4,101-108. ENGLAND, M. A. & WAKELY, J. (1977). Scanning electron microscopy of the development of the mesoderm layer in chick embryos. Anat. Embryol. 150, 291-300. EYAL-GILADI, H. & KOCHAV, S. (1976). From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. DevlBioL 49, 321-337. FONTAINE, J. & LE DOUARIN, N. M. (1977). Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. /. Embryol. exp. Morph. 41, 209-222. HAMBURGER, V. & HAMILTON, H. L. (1951). A series of normal stages in the development of the chick embryo. /. Morph. 88,49-92. HEITZMANN, H. & RICHARDS, F. M. (1974). Use of the avidin-biotin complex for specific staining of biological membranes in electron microscopy. Proc. natn. Acad. Sci., U.S.A. 71, 3537-3541. LESOT, H., OSMAN, M. & RUCH, J. V. (1981). Immunofluorescent localization of collagens, fibronectin and laminin during terminal differentiation of odontoblasts. Devi Biol. 82, 371-381. LINDER, E., VAHERI, A., RUOSLAHTI, E. & WARTIOVAARA, J. (1975). Distribution of fibroblast surface antigen in the developing chick embryo. /. exp. Med. 142, 41-49. Low, F. N. (1967). Developing boundary (basement) membranes in the chick embryo. Anat.Rec. 159,231-238. Low, F. N. (1970). Interstitial bodies in the early chick embryo. Am. J. Anat. 128, 45-56. LUNT, D. M. & SEEGMILLER, R. E. (1980). Differential localization of (35S) sulfate within ectodermal basement membrane in relation to initiation of chick limb buds. J. Embryol. exp. Morph. 60,189-199. MADRI, J. A., ROLL, F. J., FURTHMAYR, H. & FOIDART, J.-M. (1980). Ultrastructural localization of fibronectin and laminin in the basement membrane of the murine kidney. J. Cell Biol. 86,682-687. MAYER, B. W., HAY, E. D. & HYNES, R. O. (1981). Immunocytochemical localization of fibronectin in embryonic chick trunk and area vasculosa. Devi Biol. 82, 267-286. MITRANI, E. & FARBEROV, A. (1981). Fibronectin expression during the processes leading to axis formation in the chick embryo. Proc. 9th Cong. Int. Soc. Dev. Biol. MOBBS, I. G. & MCMILLAN, D. B. (1981). Transport across endodermal cells of the chick yolk sac during early stages of development. Am. J. Anat. 160, 285-308. NEWGREEN, D. & THIERY, J.-P. (1980). Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tiss. Res. 211, 269-291. NICOLET, G. (1971). Avian gastrulation. Adv. Morphogen. 9,231-262. REPESH, L. A., FURCHT, L. T. & SMITH, D. (1981). Immunocytochemical localization of fibronectin in limb tissues of the adult newt. Notophthalmus viridescens. J. Histochem. Cytochem. 29,937-945. ROSENQUIST, G. C. (1972). Endoderm movements in the chick embryo between early short streak and head process stages. /. exp. Zool. 180,95-104. SANDERS, E. J. (1973). Intercellular contact in the unincubated chick embryo. Z. Zellforsch. mikrosk. Anat. 141, 459-468. 170 E. J. SANDERS E. J. (1979). Development of the basal lamina and extracellular materials in the early chick embryo. CellTiss. Res. 198, 527-537. SANDERS, E. J. (1980). The effect of fibronectin and substratum-attached material on the spreading of chick embryo mesoderm cells in vitro. J. Cell Sci. 44, 225-242. SANDERS, E. J., BELLAIRS, R. & PORTCH, P. A. (1978). In vivo and in vitro studies on the hypoblast and definitive endoblast of avian embryos. /. Embryol. exp. Morph. 46,187-205. SILVER, M. H., FOIDART, J.-M. & PRATT, R. M. (1981). Distribution of fibronectin and collagen during mouse limb palate development. Differentiation 18, 141-149. SKUTELSKY, E., DANON, D., WILCHEK, M. & BAYER, E. A. (1977). Localization of sialyl residues on cell surfaces by affinity cytochemistry. /. Ultrastruct. Res. 61, 325-335. SOLURSH, M. & REVEL, J. P. (1978). A scanning electron microscope study of cell shape and cell appendages in the primitive streak region of the rat and chick embryo. Differentiation 11, 185-190. SPIEGEL, E., BURGER, M. & SPIEGEL, M. (1980). Fibronectin in the developing sea urchin embryo./. CellBiol. 87, 309-313. STERNBERGER, L. A. (1979). Immunocytochemistry, 2nd ed. New York: John Wiley and Sons. SANDERS, THESLEFF, I., BARRACH, H. J., FOIDART, J. M., VAHERI, A., PRATT, R. M. & MARTIN, G. R. (1981). Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Devi Biol. 81, 182-192. VAKAET, L. (1962). Some new data concerning the formation of the definitive endoblast in the chick embryo. /. Embryol. exp. Morph. 10, 38-57. VAKAET, L. (1970). Cinephotomicrographic investigations of gastrulation in the chick blastoderm. Archs Biol. (Liege) 81, 387-426. WAKELY, J. & ENGLAND, M. A. (1977). Scanning electron microscopy (SEM) of the chick embryo primitive streak. Differentiation 7, 181-186. WAKELY, J. & ENGLAND, M. A. (1979). Scanning electron microscopical and histochemical study of the structure and function of basement membranes in the early chick embryo. Proc. R. Soc. Lond. B. 206, 329-352. WARTIOVAARA, J., STENMAN, S. & VAHERI, A. (1976). Changes in expression of fibroblast surface antigen (SFA) during cytodifferentiation and heterokaryon formation. Differentiation 5, 85-89. WARTIOVAARA, J., LEIVO, I., VIRTANEN, I., VAHERI, A. & GRAHAM, C. F. (1978). Cell surface and extracellular matrix glycoprotein fibronectin: expression in embryogenesis and in teratocarcinoma differentiation. Ann. N.Y. Acad. Sci. 312, 132-141. WARTIOVAARA, J., LEIVO, I. & VAHERI, A. (1979). Expression of the cell surface-associated glycoprotein, fibronectin, in the early mouse embryo. Devi Biol. 69, 247-257. WATERMAN, R. E. & BALIAN, G. (1980). Indirect immunofluorescence staining of fibronectin associated with the floor or the foregut during formation and rupture of the oral membrane in the chick embryo. Anat. Rec. 198, 619-635. WOOD, G. S. & WARNKE, R. (1981). Supression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J. Histochem. Cytochem. 29, 1196-1204. (Received 4 January 1982)