* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Adaptive immune system wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Innate immune system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
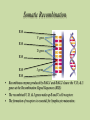
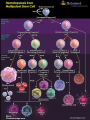
Case of Baby Joe by Susan Paran,Charmaine Pierce,Michelle Rensel,Lauren Sutton and Naomi Winterheld At birth, Baby Joe appeared to be a normal, healthy baby boy. •Parents healthy. •At four weeks of age, Joe developed an otitis media, then recurrent ear infections. •Starting at three months of age, Joe had four bouts of diarrhea which persisted for 3–5 days each time. • Joe also was not gaining weight as rapidly as was expected during this time and was diagnosed with “failure to thrive.” •At four months old, he developed another ear infection. Cultures revealed Pseudomonas aeroginosa, an opportunistic infection •Joe also had a urinary tract infection Nature or Nurture? • The underlying cause of Joe’s health problems is genetic. Although we still have to consider : • His Environment: Siblings, Daycare, Pets, etc. • His Anatomy: Shortened or narrow Eustachian tubes • Failure To Thrive? Inflammatory process affecting absorption of nutrients resulting in malnutrition No lymph nodes could be palpated in Joe’s neck or armpits. His heart, lungs, liver, and spleen were of normal size. Suspecting a problem with Joe’s immune system, Joanne ordered a complete blood count (CBC). The CBC revealed the following results: Cell Type Red blood cells Platelets Total white blood cells Neutrophils Eosinophils Monocytes Basophils Lymphocytes T cells B cells NK cells Joe’s Blood Count Normal Range 3.1x106/microliter 2.7x106–4.9x106/microliter 180,000/microliter 150,000–440,000/microliter 3500/microliter 6000–17500/microliter 2600/microliter 2000–7500/microliter 170/microliter 0–400/microliter 300/microliter 200–800/microliter 10/microliter 0–100/microliter 350/microliter 1000–4000/microliter 2% of total lymphocytes 60–80% of total lymphocytes < 1% of total lymphocytes 15–25% of total lymphocytes 90% of total lymphocytes 10–20% of total lymphocytes Can Baby Joe Make Antibodies? • Baby Joe’s B cells account for <1% of the total lymphocyte in his body. • Antibodies are produced by the plasma cell. • We need to have normal B cells that are a precursor to plasma cells in order to have normal antibody levels. How do these data explain Baby Joe’s symptoms? • Recurrent Infections – ↓ Total WBCs and lack of B cells and T cells • Inability to palpate lymph nodes & normal size spleen and liver – Lack of lymphocytes • Chronic Diarrhea -Related to his recurrent infections/ IgA deficiency Differential Diagnoses • Digeorge Syndrome • HIV • Common Variable Immunodeficiency (CVID) Can likely rule out above disorders, but not: • Severe Combined Immunodeficiency (SCID) Clinical Manifestations of SCID: • 3 most common Clinical Manifestations: – Severe, recurrent infections – Chronic diarrhea – Failure to thrive • Absence of palpable lymphoid tissue • T Cell deficiency • B Cells can be decreased or normal • NK cells increased or normal • Recurrent Fevers • Opportunistic Infections • Usually fatal within the first year of life unless underlying deficit is corrected • • • • • • X-Linked (T-B+NK-) – cause of approx. 46% of SCID cases - mutation on gene affecting various interleukin molecules (cytokines) ADA Deficiency (T-B-NK-) – cause of approximately 17% of cases - toxic metabolites buildup causing lymphocytic death IL-7Rα (T-B+NK+) – cause of approx. 10% of cases - responsible for T-Cell maturation Jak3 Deficiency (T-B+NK-) – cause of approx. 7% of cases - enzyme that communicates cytokine information to the nucleus RAG-1 or RAG-2 Deficiency (T-B-NK+) – cause of approx. 3% of cases - gene that encodes proteins necessary for antigen receptor arrangement Artemis Deficiency (T-B-NK+) – cause of approx. 1% of cases - DNA repair factor; repairs DNA after RAG makes its’ cuts Types of Mutations • Missense Mutation • Nonsense Mutation • Silent Mutation • Frameshift Mutation Frame shift Mutation A sample of Baby Joe’s DNA was sent for sequence analysis, which revealed mutations in both copies of his RAG gene. The mutations in both genes were frame shift mutations, which resulted in a complete lack of expression of functional RAG protein. • Recombinase-Activating Gene (RAG-1 or RAG-2) • Antigen Receptor Site Formation Somatic Recombination RSS V gene RSS D gene RSS RSS J gene RSS • Recombinase enzyme produced by RAG-1 and RAG-2 cleave the V, D, & J genes at the Recombination Signal Sequences (RSS) • The recombined V, D, & J genes make up B and T cell receptors • The formation of receptors is essential for lymphocyte maturation Stages of Hematopoiesis Treatment Options • IVIG Transfusion • Hematopoietic Stem Cell Transplants/BMT • Gene Therapy IVIG Transfusions • Healthy effective antibodies pooled from multiple donors. • Usually given every 3-4 weeks. • Expensive!!! Hematopoietic Stem Cell or BM Transplant • Bone Marrow Transplant (BMT) is the widely used cure. • Abnormal lymphocytes replaced by immunocompetent cells. • Success rates 90% or higher with close HLA matched donor. – Haploidentical match (from parent) with 78% success rate. • BMT for SCID doesn’t require preparative chemotherapy. Bone Marrow Transplant Cont. • T Cell depleted donor cells decrease chance of Graft Versus Host Disease (GVHD). • May not generate normal B Cell function. • Higher success rates when performed early in life. Umbilical Cord Blood • Umbilical cord blood stem cell transplantation has several advantages. – More readily available vs. obtaining bone marrow – Lower risk of viral infection transmission – NO risk to donor – Lower risk for GVHD Gene Therapy • Not currently a viable option unless a failure with BMT Epidemiology • Incidence of SCID is estimated to be 1:50,000 to 1: 500,000 live births • About half of all cases of SCID are X-linked • Other cases, like RAG-deficiency in Joe, are autosomal recessive (Bonilla, 2011) Parental Education • Neither parents, caregivers, nor patient should receive live vaccines • Encourage parents to join a support group • Keep child away from large crowds and areas where infection is likely. • Have child wear a mask if you bring into public places • Any small illness acquired by a child with SCID merits medical attention • Frequent hand washing is essential • Limited Contact with Relatives Parental Education Autosomal Recessive Inheritance Carrier Father Carrier Mother S S s SS (normal) Ss (carrier) Legend: S (normal gene) s (carrier) s Ss (carrier) ss (affected with disease) Clinical Questions In what type of mutation do one or more base pairs on a DNA chain get deleted or inserted? A. Missense Mutation B. Nonsense Mutation C.Frameshift Mutation D.Silent Mutation What is/are the most common clinical manifestation(s) of SCID? • • • • • A. Chronic Diarrhea B. Failure to Thrive C. High WBC count D. Recurrent Infections E. Only A, B, D True or False? If both your parents carry an autosomal recessive mutation, you have a 25% chance of developing the disease associated with the mutation Higher survival rates are seen in stem cell transplants with Haploidentical matches from a parent donor when compared to that of the HLA match of a sibling. • True or False? What advantages would umbilical cord blood stem cell transplantation offer in comparison to BMT? a) No chance for Graft versus host disease b) No risk to donor c) No HLA markers on umbilical cord blood stem cells so universally transferrable D) Can be transplanted at birth to avoid risk associated with acquired disease David Vetter 1971-1983 Credit: Baylor College of Medicine Photo Archives References Bonilla, F.A. (2011). Severe combined immunodeficiency (SCID): an overview. Uptodate. Retrieved 9-22-11 at http://www.uptodate.com/contents/severecombined-immunodeficiency-scid-an overview?source= search_ result&search =Scid& selected Title=1%7E94. Bonilla, F.A. (2011). Severe combined immunodeficiency (SCID): specific defects. Uptodate. Retrieved 9-20-11 at http://www.uptodate.com/contents/severecombined-immunodeficiency-scid-specificdefects? Source=search_result&search=scid&selectedTitle=2%7E94. Buckley, R. (2004). Molecular Defects in Human Severe Combined Immunodeficiency and Approaches to Immune Reconstitution. Annual Reviews of Immunology, 22, 625-655. doi:10.1146/annurev.immunol.22012703.104614 References DiPiro, J., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G, Posey, L.M (2005). Pharmacotherapy: A Pathophysiologic Approach. (6th ed.) New York:McGraw Hill. Gillepsie, S.L. (2011). Natural history and classification of pediatric HIV infection. Uptodate. Retrieved 9-22-11 at http://www.uptodate.com/contents/natural- historyand-classification- of- Pediatric-hiv-infection? Source =search _result&search=HIV+children&selectedTitle=3%7E150#H18840505.specificdefects? Source =search_result&search=Scid&selectedTitle=2%7E94. Hogan, M.B. & Wilson, N.W. (2011). Common variable immunodeficiency in children. Uptodate. Retrieved 9-22-11 at http://www.uptodate.com/contents/commonvariable-immunodeficiency-in children source= search_ result &search = CVID&selectedTitle=4%7E52. McCance, K., Huether, S., Brashers, V., Rote, N. (2010). Pathophysiology: The Biological Basis for Disease in Adults and Children. (6th ed.) Maryland Heights, MO: Mosby Elsevier References O’Donovan, D.J. (2011). Urinary tract infections in newborns. Uptodate. Retrieved 9-21-11 at http://www.uptodate.com/contents/urinary-tract-infections-in-newborns?source =search_result&search=child +uti+clinical+manifestations&selectedTitle=5%7E150. Ramphal, R. (2011). Epidemiology of pseudomonas aeuroginosa infection. Uptodate. Retrieved 9-22-11 at http://www.uptodate.com/contents/epidemiology-and-pathogenesis-ofpseudomonas-aeruginosa-infection?source=search_result&search=pseudomonas +aeroginosa&selectedTitle= 2%7E150. Seroogy, C.M. (2011). DiGeorge syndrome: pathogenesis, epidemiology and clinical manifestations. Uptodate. Retrieved 9-20-11 at http://www.uptodate.com/contents/digeorge-syndrome-pathogenesis-epidemiology-andclinical- manifestations?source=search_result&search= Digeorge+syndrome+children& selectedTitle=1%7E43#H15.