* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mitochondria consist of a matrix where three
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that
occur in specialized areas of the organism’s cells. As a basis for understanding this concept:
1. g. Students know the role of the mitochondria in making stored chemical- bond energy available to cells by
completing the breakdown of glucose to carbon dioxide.
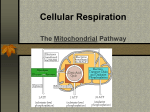
Mitochondria consist of a matrix where three-carbon fragments originating from carbohydrates are broken
down (to CO2 and water) and of the cristae where ATP is produced. Cell respiration occurs in a series of
reactions in which fats, proteins, and carbohydrates, mostly glucose, are broken down to produce carbon
dioxide, water, and energy. Most of the energy from cell respiration is converted into ATP, a substance that
powers most cell activities.
Major concepts:
1. Cells have the ability to extract stored energy from sugar molecules. Energy release begins with the
common sugar called glucose. Cells use a process called glycolysis breaks glucose down to pyruvate
with release of energy ("glycolysis" refers to taking apart sugars). Glycolysis produces energy in the
form of ATP and NADH.
2. The product of glycolysis is a three carbon sugar, pyruvate. Further breakdown occurs in a process
called the Krebs cycle. The ultimate result of the Krebs cycle is to breakdown the sugar to CO2 with
release of ATP and much more NADH.
3. NADH is a molecule that carries electrons. These electrons can be used to generate many more
molecules of ATP. Electrons released in the Krebs cycle are transfered to proteins called coenzymes. As
they pass from one coenzyme to another they are used to generate ATP molecules.
Notes:
Capturing Cell Energy
Energy has the ability to do work. All cells must convert available resources into chemical energy which may
be used by their metabolism. Even while asleep your cells are using energy. New molecules are constantly
made for growth and repair. Often a cell brings in more food energy than it can use at any one time. The cell's
chances for survival are improved if it can store the excess energy in energy storage molecules like starch or
lipids for latter use.
These storage molecules can be broken down and converted into a special energy molecule called ATP
(adenosine triphosphate). ATP is involved in nearly every metabolic step. It stores energy in a usable form.
It is the phosphate bonds that can easily release or transfer energy to nearly any metabolic process.
ATP <==> ADP + P + energy for cellular activity
Uses of Cell Energy
The use of energy by a cell involves some of the following:
•
•
•
•
•
•
•
maintaining homeostasis
moving molecules through membranes - active transport
building other molecules like protein or DNA
manufacturing new cell organelles
cell division
moving a cell or parts of the cell - cytoplasmic streaming
converting one compound into another
Some energy is always converted to a form that a cell can not use. This is called heat. Heat energy does not
change from place to place locally. It is uniform, lacking a usable gradient. This uniformity cannot be tapped by
metabolism to do work, it can only supply kinetic energy for diffusion or give a body warmth.
Three types of energy releasing pathways
Most cells are aerobic, that is they require oxygen for life. What that means is that they require oxygen to
completely break down carbohydrates to CO2. The process of oxygen dependent breakdown of carbohydrates is
called respiration (from the Latin, literally breathing). Oxygen can efficiently accept electrons from other
molecules (that is what the process we call oxidation is-the loss of electrons to another molecule). It is these
electrons which provide most of the energy of aerobic respiration. The process of respiration is occurring
rapidly in all of your cells, to create the energy necessary for their function. The cells of your body, when
deprived of oxygen for even relatively short periods, will rapidly die.
We've all seen footage of children who have fallen through the ice, and are taken, lifeless, to the ambulance.
Often they have lain deep in the water for periods of almost an hour. Their body temperature is extremely low
as a result of their immersion in the cold water. If oxygen is so important, why is it that they can often be
resuscitated and their body temperature brought back to normal, all with no long-lasting effects?
Some simple organisms can live without oxygen, and derive their energy by different mechanisms. Perhaps
more common are organisms that use fermentation to extract energy from carbohydrates. In fact, your muscles
can switch to this mechanism when oxygen is not available, for example during extremely vigorous exercise.
One problem with this mechanism is that it releases much less energy than does aerobic respiration. Muscles
will quickly tire for lack of sufficient energy. Also, the end product of fermentation is damaging to muscles.
Other organisms use a different mechanism, nonaerobic electron transport. The final electron acceptor is often
an inorganic compound found in the environment, in place of O2. Some bacteria use sulfate (SO4-), and produce
H2S, the sulfur analog of water, which we perceive as the scent of rotten eggs. Fortunately, this mechanism is
not common! All three of these mechanisms begin with a process called glycolysis (from the Greek, literally
sugar breakage). In glycolysis glucose is broken down partially, with the production of energy in the form of
ATP, and NADH (another reduced electron carrier). Glycolysis produces very little energy compared with
aerobic respiration, a little over 1/20. The anaerobic process of glycolysis, fermentation and nonaerobic
electron transport all occur in the cytoplasm, but aerobic fermentation occurs in specialized organelles, the
mitochondria.
Respiration
Six-carbon sugars are the primary source of energy for cells. The use of organic molecules for their energy is
called respiration. Respiration may or may not use oxygen. When oxygen is required the process is called
aerobic respiration. But if oxygen is not used the reparatory process is anaerobic.
Anaerobic metabolism -- glycolysis and fermentation -- is common to all life and can generate energy simply
and quickly.
Glycolysis and Fermentation
Glycolysis splits the 6-carbon glucose into 2 molecules of pyruvate, a three carbon molecule illustrated below.
The molecular formula of pyruvate is C3H3O3-. Pyruvate is the salt of pyruvic acid. The only difference is the
addition of a hydrogen in the neutral pyruvic acid molecule -- C3H4O3.
ATP is involved in glycolysis. This 10 step process makes 4 ATP’s by combining ADP with phosphate.
ADP + P ---> ATP or
A-P~P + P ----> A-P~P~P
However 2 ATP molecules are needed to "energize or prime" glucose for the remaining steps so a net of only 2
ATP’s are produced.
The final product of the reaction is pyruvic acid, or pyruvate, a three carbon organic acid. It is the starting
material for each of the three subsequent pathways.
Bottom line: one glucose is converted to two pyruvates with the production of 2 ATP and 2 NADH:
Glucose + 2 NAD+ + 2 ADP + 2 Pi -----> 2 pyruvate + 2 NADH + 2 ATP + 2 H2O
Pyruvate is involved in two types of fermentation.
Yeast and a great many other organisms convert pyruvate into ethyl alcohol and carbon dioxide (CO2) in a
process called alcoholic fermentation. Ethyl alcohol is illustrated below.
Humans and animals in general, rather than getting drunk when they exercise convert pyruvate into lactic acid
(or lactate). This is called lactic acid fermentation. See the illustration below -- note the similarity to pyruvate.
Fermentation is essential to anaerobic organisms because it recycles the coenzyme NADH formed during
glycolysis back to NAD+. Otherwise glycolysis would stop when it ran out of NAD+.
Lactic acid or lactate increases a person's sensitivity to pain. When you overwork muscles lactic acid is formed,
and the associated pain is nature's way of warning you to stop what you are doing. In the first place, the lack of
ATP causes the muscles to tire.
In the second place, the presence of the lactate causes some of the pain which occurs in periods of extreme
exercise.
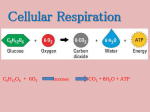
Aerobic Respiration
Aerobic respiration uses oxygen to completely break down glucose, releasing and converting as much energy as
possible in the form of ATP. The overall efficiency of aerobic respiration is almost 36%, while the efficiency of
glycolysis is only 2%.
Aerobic respiration may be summarized using the following equation:
C6H12O6 + 6O2 -----> 6CO2 + 6H2O + energy (36 ATP)
Aerobic respiration begins when glycolysis ends. It involves 3 phases.
1. Pyruvate is modified and combined with a 4-carbon molecule in mitochondria to produce 1 citric acid, a
molecule of CO2, and a molecule of NADH.
2. The citric acid molecule takes part in an energy releasing process called the citric acid cycle. The cycle
which takes place in the matrix of the mitochondria, generates 2 molecules of CO2, 3 molecules of NADH, 1
FADH2 and 1 ATP.
3. NADH and FADH2 are coenzymes needed to take electrons and H+ ions to the electron transport chain
embedded in the inner membrane of mitochondria -- cristae. The electrons are used to make 18 ATP molecules
per glucose. This is where the efficiency of aerobic respiration is evident. The reduced coenzymes produced by
the Krebs cycle are immediately used to create ATP. The electrons carried on these molecules are passed down
an electron transport chain (resembling that between photosystem II and photosystem II)
As the electrons pass down the chain protons (H+) are pumped out of the inner compartment into the outer
compartment of the mitochondrion. The ultimate electron acceptor is O2 which is reduced to H2O. Transport of
protons creates a large pH gradient across the membrane, and an electric potential caused by the separation of
charged ions (+ on the outside and - on the inside)
The proton gradient is used, as in photosynthesis, to generate ATP. A protein in the inner membrane allows the
protons to travel down the gradient into the inner compartment. The energy of passage of each proton is used to
phosphorylate ADP to produce ATP. Electrons from NADH ultimately result in the production of 3 ATP.
FADH2 enters part of the way down the electron transport chain and is converted into only 2 ATP. The
electrons on the 2 NADH produced by glycolysis are usually transported to FAD in the mitochondrion, giving
another 4 ATP per glucose. Because one glucose molecule produces 2 pyruvates the steps above should be
doubled.