* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download felix may 2nd year neuroscience Evaluation of the effect of chronic
Neuroendocrine tumor wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Bioidentical hormone replacement therapy wikipedia , lookup
Growth hormone therapy wikipedia , lookup
Hypopituitarism wikipedia , lookup
Hypothyroidism wikipedia , lookup
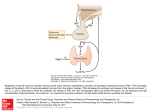
felix may 2nd year neuroscience Evaluation of the effect of chronic treatment with methimazole on pituitary-thyroid axis function in the rat Aims To analyse data from an experiment performed in rats. Levels of thyroxine (T4) were assayed from the blood and thyroid glands, while thyrotropin-releasing hormone levels were assayed from the hypothalamus and brainstem. There were two groups of animals, one treated with methimazole for five days, and a control group. Introduction Assaying blood levels of hormones is a vital diagnostic tool used in clinics throughout the world. The development of radioimmunoassays (RIA) for detecting pico-molar concentrations of hormones has transformed the study of endocrinology. Today simple assay preperations are available for physicians to assess a patient’s thyroxine levels, without access to lab facilities. Thyroid hormones play a crucial role in homeostatic regulation, affecting the metabolic functioning of every cell in an animal. Development and growth are modulated by thyroid hormones, as is the sympathetic nervous system. Dysfunction of the pituitary-thyroid axis causes and exacerbates many clinical conditions, including Grave’s disease (an autoimmune hyperthyroidism) and Myxedema (a hypothyroid disorder, where vasopressin and heart function are also affected, causing oedema). Thyroid function is also interwoven with the endocrine control of the female reproductive system… indeed Grave’s disease is symptomatically similar to normal thyroid changes that occur in pregnancy, making diagnosis tricky. Two thyroid hormones, thyroxine and tri-iodothyronine (T4 and T3), are secreted from follicular cells in the para thyroid glands found around the larynx. Thyroid hormone synthesis is stimulated by ciculating thyroid-stimulating hormone (TSH) which is secreted from thyrotroph cells in the anterior pituitary. TSH secretion is controlled by thyrotropin releasing hormone (TRH) released into the portal vessels from the paraventricular nucleus of the hypothalamus. TRH secretion is increased by stimuli such as cold trauma and high food intake. Both TSH and TRH secreting cells are inhibited by circulating thyroid hormones, providing negative feedback to the axis. T3 and T4 exert their effects through nuclear receptors, as well as some mitochondrial and membrane targets. Within the cell T4 is converted to T3, thus T4 is a prohormone. In the blood both hormones are 99.9% bound to thyroxine-binding globulin and prealbumin. Control of thyroid secretions maintains the free hormone levels independent of protein binding, a thus free hormone concentrations are a direct indication of thyroid status. Thyroid homornes are lipid soluble and can cross cell membranes by diffusion. Methimazole is an antithyroid drug that inhibits tyrosine peroxidase, and thus the organification of iodine into the precursors monoiodotyrosine and diodotyrosine, preventing the biosynthesis of thyroid hormones. Methods Rat TSH has a different amino acid sequence in its protein subunit to that in human TSH, and assays for it are unavailable, so TRH was assayed as an indirect indication of pituitary-thyroid function. Rats were treated with methimazole for 5 days at 40mg/kg or with a saline as controls. Over this period the drug had time to equilibrate and the body to respond fully. Five animals were in each treatment group, enough to provide reliable results, without excessive sacrifice. Free plasma thyroxine (T 4) was assayed from mixed arterio-venous blood collected from the animals in heparin coated tubes (to prevent blood clotting). T 4 is assayed rather than T3 as it has a longer half-life and is present in higher concentrations. Free T4 in the thyroid was assayed from a preperation of the thyroid gland. The tissue was dissected and sonicated to lyse the cells and homogenise their contents. Methimazole was added to the veronal buffer to prevent furthur production of thyroid hormones. The suspension was then centrifuged to remove cell debris. The Radio-immuno assay for free T 4 used a radiolabelled antibody and hormone bound to metallic beads. Antibodies that bind to the free T 4 will remain in solution whereas those that bind the the beaded hormone will be centrifuged out. The remaining activity gives a direct determination of the content of T 4. Levels of TRH in the brain were also assayed… Both the hypothalamus and brainstem were prepared by dissection and sonication in 90% methanol, then centrifuged. Extracting TRH from blood would yield tiny quantities, as the hormone usually has to travel no futhur than down the portal veins to the pituitary to exert it effects. TRH levels were radioassayed using radiolabelled 125I-TRH, in the standard competition for antibody binding with the unknown quantity of natural TRH. Standard curves for the free T4 and TRH assays were drawn using data produced with known quantities of radioactive and natural ligands. Using these curves the concentrations of hormones from the experiment could be extrapolated. Results Blood free T4 mean SEM methimazole saline 180 ±56.4 600 ±81.2 Hypothalamus TRH mean SEM methimazole saline 481 ±69.6 77.5 ±5.6 unpaired t-test P (P < 0.05) 0.0081 significant unpaired t-test P (P < 0.05) 0.0044 significant Discussion From the results it can be seen that in rats who received methimazole treatment thyroxine levels were significantly lower than in the saline treated group, both in blood and thyroid. Upon t-testing the difference between groups for blood T 4 it was confirmed the the results were significantly different. Concentrations of T4 in the blood were proportionally lower than in the thyroid for both methimazole and control groups. This is expected as the thyroid is the site of synthesis for T 4 and contains large stores of the hormones. TRH in the hypothalamus was significantly higher in the methizamole groups than the control rats. The differences between groups for hypothalamic TRH concentrations are obviously great, and this was confirmed by the t-test. However TRH levels in the brainstem were largely unaffected by methimazole. Within the brainstem, especially the dorsal raphe nuclei, TRH occurs as a neuropeptide. It is likely that TRH is synthesised by neurons in the brainstem independent of the pituito-thyroid axis’ state. Methimazole reduced the circulating concentrations of free T 4 (and presumably T3) as shown in the experiment. The hypothalamus and the pituitary will respond to a fall in thyroid hormone concentrations in the blood by releasing greater amounts of TRH and TSH, respectively. This experiment clearly demonstarated the rise in TRH that would be predicted from the understanding of negative feedback in the pituito-thyroid axis. Treatment with anti-thyroid drugs aims to reduce the effects of thyroid hormones in the body, such as in cases of hyperthyroidism. Methizamole acts to prevent the synthesis of tyrosine radicals, an essential step in the formation of iodotyrosine, the precursor to thyroid hormones. Other potential targets for antithyroid drugs include TRH and TSH receptors, although clinical medicines that act on those targets are yet to be introduced. Iodine is given in cases of hyperthyroidism as it (almost paradoxically) inhibit’s the secretion of thyroid hormones and sends the thyroid glands into a semi-dormant state. This treatment is best for acute hyperthyroidism as its effectiveness declines rapidly after a week or so. Radioactive iodine is also given, but for more chronic depression of thyroid function. 131I is absorbed and concentrated in the thyroid glands where it decomposes, after two months reaching effectively zero activity. The beta radiation emitted during decomposition ionises the follicular cells, which is highly cytotoxic. Thus the gland is directly restricted by culling a portion of its cells. This experiment used a small number of animals to aquire results, and thus could be criticised for having insignificant sample numbers. However the clear trends in results and coherence with the literature suggest that the number of animals was enough for this purpose. References: Antepartal nursing management of Grave's disease - B. Karacic - Journal of Obstetric, Gynecologic, and Neonatal Nursing, Vol 15, Issue 3 214-218 hypothalamic-pituitary-thyroid axis and the female reproductive system - Doufas AG, Mastorakos G. - Ann N Y Acad Sci. 2000;900:65-76 Thyroxine treatment induces upregulation of renin-angiotensin-aldosterone system due to decreasing effective plasma volume in patients with primary myxoedema - Cheol Whee Park, Young Shin Shin, Seog Ju Ahn, Suk Young Kim, Eui Jin Choi, Yoon Sik Chang and Byung Kee Bang - Nephrol Dial Transplant (2001) 16: 1799-1806 Pharmacology 5th edition - Rang, Dale, Ritter, Moore The Merck Manual of Diagnosis and Therapy, Chapter 8. Thyroid Disorders