* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download aspartate Antagonists on a Multiple Schedule of Ethanol and
Pharmacokinetics wikipedia , lookup
Prescription costs wikipedia , lookup
Polysubstance dependence wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
NMDA receptor wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
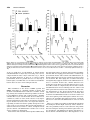
0022-3565/97/2803-1250$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Copyright © 1997 by The American Society for Pharmacology and Experimental Therapeutics JPET 280:1250 –1260, 1997 Vol. 280, No. 3 Printed in U.S.A. Effects of g-Aminobutyric Acid Agonists and N-Methyl-Daspartate Antagonists on a Multiple Schedule of Ethanol and Saccharin Self-administration in Rats1 KEITH L. SHELTON2,3 and ROBERT L. BALSTER Department of Pharmacology and Toxicology, Medical College of Virginia, Virginia Commonwealth University, Richmond, Virginia Accepted for publication November 19, 1996 A diverse group of neurotransmitter systems are affected by ethanol, and the roles of these systems in the production of the pharmacological and behavioral effects of ethanol are beginning to be understood. Relatively recently, the actions of ethanol on the NMDA subtype of glutamate receptors and the GABAa/benzodiazepine receptor system have received considerable attention (Grant, 1994). It is known that ethanol potentiates the effect of GABA and the GABAa agonist muscimol in several in vitro functional assays (Mehta and Ticku, 1988; Takada et al., 1989). There is also emerging evidence that ethanol has a number of effects on the NMDA receptor complex at physiologically relevant concentrations (Gonzales and Woodward, 1990; Lovenger et al., 1989; Nie et al., 1994). At the behavioral level, ethanol intoxication, tolerance and Received for publication June 25, 1996. 1 This research was supported by National Institute on Alcoholism and Alcohol Abuse Grant AA08437 and National Institute on Drug Abuse Grant DA01442. 2 Supported by National Institutes of Health Training Fellowship AA05357. 3 Present address: Department of Psychiatry and Behavioral Sciences, M.S.I., University of Texas Health Science Center at Houston, 1300 Moursund, Houston, TX 77030. ethanol selectively decreased ethanol self-administration without altering saccharin self-administration. The competitive NMDA antagonist CPPene (D-3-(2-carboxypiperazine-4-yl)-1propenyl-1-phosphonic acid [SDZ EAA 494]) and the noncompetitive NMDA antagonist phencyclidine decreased both ethanol and saccharin self-administration. The GABA agonists pentobarbital and diazepam also failed to reduce ethanol selfadministration, relative to saccharin. Although these results do not support the hypothesis that antagonism of the NMDA receptor system or activation of the GABA receptor system can selectively modify ethanol-reinforced responding, they identify important issues for designing the best strategies to be used to assess selective drug effects on ethanol self-administration. dependence may also have both a GABAergic component and a NMDA component. GABA agonists potentiate ethanol-induced sedation (Lilijequist and Engel, 1982) and, in a similar fashion, NMDA antagonists increase the potency of ethanol for inhibiting the righting reflex in mice (Daniell, 1990) and decreasing locomotor activity in rats (Robledo et al., 1991). Barbiturates and benzodiazepine also exhibit cross-tolerance (Rosenberg et al., 1983) and cross-dependence (Cooper et al., 1979) with ethanol, whereas noncompetitive NMDA antagonists attenuate alcohol withdrawal seizures (Morrisett et al., 1990) and enhance the behavioral and toxic effects of ethanol (Stone and Forney, 1977; Wessinger and Balster, 1987). In yet another line of investigation, drug discrimination studies have shown that ethanol and certain GABA agonists and NMDA antagonists have similar interoceptive stimulus properties (Grant and Colombo, 1992; Grant et al., 1991; Kubena and Barry, 1969; Sanger, 1993; Shelton and Balster, 1994; Winter, 1975) . Both the GABAa/benzodiazepine receptor system and, to a lesser extent, the NMDA receptor system have been implicated as modulatory substrates for ethanol self-administration. GABA-transaminase inhibitors, GABAb agonists ABBREVIATIONS: AP-5, 2-amino-5-phosphonopentanoic acid; AP-7, 2-amino-7-phosphonoheptanoic acid; CPPene, D-3-(2-carboxypiperazine4-yl)-1-propenyl-1-[phosphonic acid [SDZ EAA 494]; FR, fixed ratio; GABA, g-aminobutyric acid; NMDA, N-methyl-d-aspartate; PCP, phencyclidine. 1250 Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 ABSTRACT Recently, it has been shown at both the cellular and behavioral levels that ethanol has effects on the N-methyl-D-aspartate (NMDA) and g-aminobutyric acid (GABA)a receptor systems, leading to the possibility that the reinforcing effects of ethanol may be, at least partially, mediated via these receptor ionophores. In this study, a multiple schedule of ethanol and saccharin self-administration was used to study that possibility. Adult male Long-Evans rats were trained during 1-hr sessions to press on two different levers for 10% (w/v) ethanol and 0.1% (w/v) saccharin solutions, under an alternating 5-min, fixedratio-4 schedule of liquid availability. After training, tests were conducted with ethanol, NMDA antagonists and GABA agonists given before six consecutive sessions. Pretreatment with 1997 1251 assess the ability of the procedure to detect selective drug effects, because one might predict that noncontingent ethanol would selectively decrease ethanol-maintained responding. Methods Subjects. Twelve experimentally naive, adult, male, Long-Evans hooded rats (Harlan Sprague Dawley, Indianapolis, IN), weighing 275 to 300 g at the beginning of the study, were used as experimental subjects. The rats were individually housed in standard hanging wire rodent cages. A 12-hr light/dark cycle was in effect for the duration of the study. The rats were food-restricted to 15 g of Agway rodent chow per day, which was available after the experimental sessions, with the exceptions noted for the ethanol-induction phase of the study. Rats reached an average weight of 350 g by the end of the experiment. Apparatus. Experimental sessions were conducted in a separate room in two-lever operant conditioning chambers (BRS/LVE, Beltsville, MD), each of which had been fitted with a custom-built front panel (Fabco, Albany, TX) containing two solenoid-operated liquiddelivery devices with two associated rodent response levers and 8-W stimulus lights (Coulbourn Instruments, Allentown, PA). The solenoid value system allowed small amounts of liquid (0.05 ml/delivery) to be accurately metered into 0.15-ml liquid cups located on the front interior wall of each cage. Liquid was supplied to each solenoid value through 3/8-inch Tygon tubing (Norton Performance Plastics, Akron, OH) attached to suspended 250-ml aspirator bottles (Corning, Corning, NY). The chambers were individually housed in sound-attenuated and ventilated cubicles (BRS/LVE). Experimental sessions and data recording were accomplished using an IBM-compatible 486DX2 computer system (Win Laboratories, Manassas, VA), running Med-state operant conditioning software. Smart-control interface equipment linked the computer and operant chambers (Med Associates, Albans, VT). Drugs. Dehydrated 100% ethyl alcohol (Pharmco, Bayonne, NJ) was obtained from the Medical College of Virginia hospital pharmacy and diluted with tap water into concentrations of 1, 3, 6 and 10% (w/v) for the drinking solutions. The same 100% ethanol was diluted in saline for the pretreatment injections. Saccharin HCl (Sigma Chemicals) was diluted in tap water to a concentration of 0.1% (w/v). PCP HCl (National Institute on Drug Abuse, Rockville, MD) was dissolved to injection concentrations with sterile saline. Sodium pentobarbital (Anthony Products, Arcadia, CA) and diazepam HCl (Elkins-Sinn, Inc., Cherry Hill, NJ) were diluted from a commercial injection solution into a vehicle consisting of 50% sterile water, 40% propylene glycol and 10% ethanol. CPPene (Sandoz Research Institute, Berne, Switzerland) was dissolved in sterile saline, and the pH was adjusted to between 6 and 7 by the addition of sodium hydroxide. All drugs and vehicles were sterile-filtered (Millipore filters, 0.2 mm; Gellman) before use. Presession injections were given i.p. at a volume of 1 ml/kg, with the exception of ethanol, which was administered i.p. at a volume of 10 ml/kg. Ethanol (180, 560, 1000 and 1560 mg/kg), PCP (1, 2 and 4 mg/kg) and pentobarbital (3, 10 and 20 mg/kg) were injected 15 min before the start of the session. Diazepam (1, 3 and 5.6 mg/kg) was injected 30 min before the session, and CPPene (1, 3 and 5.6 mg/kg) was injected 60 min before the session. Training and testing procedure. The animals were tested 5 days per week (Monday through Friday), between 8:00 A.M. and 12:00 noon. To promote operant responding for liquid, the animals were initially maintained in a water-deprived state for 20 hr before the start of each session. In addition, 5 g of each animal’s daily food allotment was placed in the home cage 15 min before each session. Over the course of 20 sessions, the animals were trained to respond for water under a multiple schedule for 0.05-ml water deliveries. Each session lasted for 1 hr and consisted of 12 (5-min) periods of water availability. Every 5 min, the active liquid delivery device was Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 (Daoust et al., 1987), picrotoxin-site ligands (Rassnick et al., 1993a) and GABA metabolites (Fadda et al., 1983; Gallimberti et al., 1989) have all been shown to decrease ethanol intake in a number of models. Several benzodiazepines have also been tested for their ability to affect ethanol self-administration; the findings from these studies are mixed, with both positive (Samson and Grant, 1985) and negative (Daoust et al., 1987; Rassnick et al., 1993a) results being obtained. The benzodiazepine partial inverse agonist Ro15– 4513 decreases ethanol intake in choice as well as in forced drinking studies (June et al., 1992; McBride et al., 1988; Rassnick et al., 1993a; Samson et al., 1987, 1989). The role of the NMDA receptor site in modulating ethanol self-administration has not been well characterized. To date, there are only two published reports concerning the ability of NMDA antagonists to attenuate ethanol self-administration. Both of these studies came from the same laboratory group, and they used similar experimental designs. These preliminary results indicate that injections of the competitive NMDA antagonists 2-amino-5-phosphonopentanoic acid (AP-5) and 2-amino-7-phosphonoheptanoic acid (AP-7) directly into the nucleus accumbens attenuate operant responding for 10% ethanol without affecting baseline levels of water self-administration (Rassnick et al., 1992a,b). Many studies have examined drug effects on ethanol selfadministration behavior; however, relatively few experiments have attempted to assess the selectivity of pretreatment drug effects for ethanol self-administration, compared with other reinforcers. Clearly, pharmacologically useful treatment drugs for ethanol abuse should exhibit some selectivity for attenuating drug-reinforced responding without altering other behaviors. The more commonly used methods of assessing the effects of pretreatment drugs on a self-administration base line, such as bottle drinking and simple operant schedules, are generally of limited utility in separating any attenuation of reinforcing effects from nonspecific behavioral disruption, which is often caused by these pretreatment compounds. A number of methods have been devised to overcome this limitation, such as the use of a second control group of subjects self-administering a non-drug reinforcer (Hubner and Koob, 1990; Rassnick et al., 1993a; Samson et al., 1989) or the concurrent availability of water along with drug (Rassnick et al., 1993b). In studies using the latter method, rates of water self-administration are often very low compared with drug; therefore, rate-disruptive drug effects would be difficult to assess due to the lack of a similar operant baseline. Overall, these strategies provide some assessment of selectivity but are far from ideal controls. A number of i.v. drug self-administration studies in nonhuman primates have addressed the issue of pretreatment selectivity by training animals under multiple schedules in which alternating periods of drug and food availability are used to assess nonspecific drug effects (Aigner and Balster, 1979; Mello et al., 1993; Woolverton and Virus, 1989). It was our goal to develop a multiple schedule of oral ethanol and saccharin self-administration in rats similar to that previously used to assess selectivity of drug effects on i.v. selfadministration in monkeys. We then tested a number of drugs acting as GABAa/benzodiazepine receptor agonists or NMDA antagonists for their ability to alter ethanol selfadministration at doses that did not effect saccharin selfadministration. Ethanol pretreatment was also studied to Ethanol Self-Administration 1252 Shelton and Balster Results Ethanol and water self-administration. The data from the 10 sessions of 10% ethanol and water self-administration are shown in figure 1. During the first two self-administration sessions, rates of ethanol and water self-administration were similar. Over the next several two-session blocks, ethanol self-administration steadily increased to a high of 36 deliveries, whereas water self-administration decreased nearly to zero levels. These results clearly show that the delivery of 10% ethanol served as a reinforcer under the multiple schedule. Effects of ethanol pretreatment on ethanol and saccharin self-administration. The results of ethanol pretreatment on total session rates of ethanol and saccharin self-administration are shown in figure 2. A total of nine rats met the ethanol and saccharin delivery performance criteria for inclusion in this dose-effect curve. Ethanol pretreatment dose-dependently suppressed ethanol self-administration (fig. 2, top left). The 1000 mg/kg and 1560 mg/kg doses of ethanol significantly [t(8) 5 4.95 and t(8) 5 3.558, respectively] reduced ethanol responding relative to saline control sessions. Ethanol preinjections also significantly affected saccharin responding; however, the effects did not appear to be dose-related (fig. 2, top right). At the 180 mg/kg and 560 mg/kg doses of ethanol, small but statistically significant increases [t(8) 5 22.90 and t(8) 5 22.75, respectively] in saccharin self-administration were observed. The 1000 mg/kg ethanol dose significantly decreased [t(8) 5 6.38] saccharin deliveries relative to the preceding saline control. No effect on saccharin self-administration was observed at the 1560 mg/kg ethanol pretreatment dose. The daily means of ethanol and saccharin deliveries during the ethanol pretreatment dose-effect determination are also shown in figure 2. Saccharin self-administration did not reach a stable baseline level until well into the ethanol doseeffect curve (fig. 2, bottom right). When the data are shown in this way, it is clear that the ethanol-produced increases in saccharin self-administration are probably an artifact of the steady increases in rates of saccharin-reinforced responding over this period, independent of ethanol pretreatment. Eth- Fig. 1. Comparison of ethanol (EtOH) and water self-administration under a multiple schedule of alternating 5-min periods of ethanol and water availability. Deliveries (mean 6 S.E.M.) of 10% (w/v) ethanol (f) and water (E) over the course of five two-session blocks are shown. Each data point is a group mean and is composed of the mean deliveries over two sessions for each subject. Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 alternated (i.e., left, right, left, etc.). Each period of access from a device was signaled by the illumination of an amber stimulus light directly above the spout cup. Only responses on the lever associated with that device produced fluid deliveries. Responding on the other lever was recorded but had no scheduled consequences. The first delivery device to be active (left or right) was alternated daily. When responding had stabilized under a FR-1 schedule for water delivery, the FR size was increased over a period of 10 days to FR-4. After FR-4 responding for water was obtained, increasing concentrations of ethanol were introduced into both delivery devices. Every 10 sessions, the ethanol concentration was increased in a stepwise fashion (1, 3, 6 and 10%, w/v) until the rats were responding for a 10% ethanol solution. At that point, the water restriction was gradually discontinued over a period of 10 days. After that, the presession feeding was also discontinued over a period of 10 sessions. The 10% ethanol in one of the two delivery systems was then replaced with water for 10 sessions to assess the reinforcing effects of ethanol in alternate components. After this initial test for ethanol reinforced responding, a 0.1% saccharin solution replaced water in alternating components of each daily self-administration session. Because saccharin is a potent reinforcer in rodents, no training was necessary to initiate operant responding for saccharin deliveries. The sides from which ethanol and saccharin were available remained constant throughout the study. To balance the time periods of ethanol and saccharin access, the side that was active at the start of the session was alternated daily (i.e., left, right, left, right), resulting in ethanol being available first in half of the sessions and saccharin being available first in half of the sessions. The multiple schedule of 10% ethanol and 0.1% saccharin was then tested for 10 sessions, to allow the self-administration of both solutions to stabilize. Pretreatment tests were then conducted with various doses of ethanol, PCP, CPPene, diazepam and pentobarbital, in that order. Each drug test block consisted of six daily drug injections at each dose. Every block of drug sessions was preceded by six sessions of daily vehicle injections. Drug doses were administered in ascending order for all of the drugs tested. Data analysis and inclusion criteria. The first two sessions of each six-session block of drug pretreatments were discarded from the data analysis based on the a priori assumption that these sessions were likely to show behavior in transition. The final four sessions were used for data analysis purposes. Both correct and incorrect responses and liquid deliveries were collected for each 5-min segment, as well as for the total session. Changes in overall rates of ethanol and saccharin self-administration, as well as changes in the within-session distribution of responding, were determined from the number of fluid deliveries. Separate two-tailed paired t tests were performed for each drug dose, comparing each drug dose to the saline control block immediately preceding that dose. Individual data points for these statistical analyses consisted of the mean of each animal’s last 4 days at each dose of pretreament drug and the mean of the last 4 days of each saline control block. Separate t tests were performed for both ethanol and saccharin self-administration data. The criterion for statistically significant effects was set at the P , .05 level. Only animals that exhibited a mean of at least 20 ethanol and 20 saccharin deliveries during the final four saline-injection control sessions before each dose-effect curve were used for that dose-effect curve. Animals that exhibited some ethanol self-administration but failed to reach the inclusion criteria received additional training in the event that their level of ethanol self-administration increased sufficiently to reach the inclusion criteria for the next dose-effect curve. Animals that consistently exhibited no responding or very low levels of responding for ethanol were removed from the study. Vol. 280 1997 Ethanol Self-Administration 1253 anol pretreatment at both the 560 mg/kg and 1000 mg/kg doses resulted in a progressive decrease in ethanol self-administration over sessions (fig. 2, bottom left). The data also show that ethanol deliveries quickly returned to baseline levels after cessation of ethanol pretreatment. Figure 3 illustrates the within-session pattern of responding for ethanol and saccharin. Plotted in figure 3 are the mean ethanol deliveries (fig. 3, left) and saccharin deliveries (fig. 3, right) in each 5-min session component, averaged across the last 4 days of each 6-day block of ethanol and saline pretreatment. The within-session patterns of control ethanol and saccharin self-administration show similar numbers of ethanol and saccharin deliveries during the initial 5-min period of availability, followed by a marked decrease in ethanol self-administration in later components. Saccharin self-administration also decreased over the course of the session, but to a much lesser degree than ethanol self-administration. As was the case with the session totals, ethanol pretreatment dose-dependently suppressed ethanol drinking during the first 5-min component (fig. 3, left). Conversely, ethanol pretreatment had little effect on saccharin intake during the first 5-min bin. Ethanol pretreatment at doses of 560 and 1560 mg/kg did, however, have rate-increasing effects on saccharin self-administration, which were more apparent in later bins. These increases may also be a result of the steadily increasing saccharin baseline and not a consequence of ethanol pretreament itself. Effects of PCP pretreatment on ethanol and saccharin self-administration. Seven rats met criteria for inclusion into the PCP dose-effect curve. The effects of PCP pretreatment on session totals for ethanol and saccharin selfadministration are shown in figure 4. Doses of 1 and 2 mg/kg PCP did not have any significant effect on either ethanol or saccharin deliveries, whereas 4 mg/kg decreased both. The 4 mg/kg dose of PCP significantly [t(6) 5 2.802] suppressed ethanol responding (fig. 4, top left). Saccharin self-administration was likewise significantly [t(6) 5 2.735] decreased by 4 mg/kg PCP (fig. 4, top right). Figure 4 also shows the daily means for both ethanol (fig. 4, bottom left) and saccharin (fig. 4, bottom right) deliveries. There were no apparent trends over days for control rates of either ethanol or saccharin self-administration across this phase of the study. In addition, there were no noticeable trends within blocks of PCP pretreatment at 1 and 2 mg/kg Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 2. Effects of noncontingent ethanol (EtOH) pretreatment on ethanol and saccharin self-administration. Top, ethanol (left) and saccharin (right) deliveries after pretreatment with ethanol (f) and deliveries during pretest saline control sessions (M). Bottom, left, daily means of ethanol deliveries during noncontingent ethanol administration (f) and during pretest saline control sessions (M); right, daily means of saccharin deliveries during noncontingent ethanol administration (F) and during pretest saline control sessions (E). Points shown are the number of deliveries (mean 6 S.E.M.) of ethanol and saccharin received in the last four sessions of a six-session block at each condition. *, statistically significant effects (P , .05). 1254 Shelton and Balster Vol. 280 PCP, nor was there any evidence of suppression of ethanol or saccharin self-administration until pretreatment with the 4 mg/kg dose of PCP. At this dose, rates of both ethanol and saccharin self-administration decreased over days of PCP pretreatment. Effects of CPPene pretreatment on ethanol and saccharin self-administration. A total of 10 rats were used for the determination of the CPPene dose-effect curve. The results of CPPene pretreatment on the four-session means of ethanol and saccharin deliveries are shown in figure 5 (top). The 1 mg/kg dose of CPPene had no effect on ethanol intake (fig. 5, top left); however, this dose of CPPene slightly but significantly [t(9) 5 2.29] suppressed saccharin self-administration (fig. 5, top right). The 3 mg/kg dose of CPPene had a nonselective effect on ethanol and saccharin self-administration, significantly suppressing ethanol [t(9) 5 4.63] and saccharin [t(9) 5 6.28] self-administration. The effects of 5.6 mg/kg CPPene were similar to, but more pronounced than, those of 3 mg/kg. Ethanol deliveries were significantly suppressed [t(9) 5 5.16] to ,25% of control levels. Saccharin deliveries were also greatly reduced [t(9) 5 5.08, P , .05], by .64%, compared with pretest saline control levels. The single-session means of ethanol and saccharin deliveries over successive days also clearly show the pronounced dose-related effects of CPPene on both saccharin and ethanol self-administration (fig. 5, bottom). Although there was some variability in the daily data, the effects of CPPene on ethanol and saccharin self-administration were consistent, in that the lowest number of both ethanol and saccharin deliveries under saline control conditions were higher than the greatest number of deliveries at the 3 and 5.6 mg/kg doses of CPPene. The effects of CPPene on ethanol self-administration progressively increased over days of CPPene administration, whereas the effects of CPPene on saccharin self-administration did not show a trend across days. As was the case with PCP, baseline levels of ethanol and saccharin self-adminis- Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 3. Within-session time-course showing the results of ethanol (EtOH) pretreatments on successive 5-min segments of ethanol (left) and saccharin (right) self-administration, averaged across the last four of six test sessions. u, ethanol and saccharin deliveries (mean 6 S.E.M.) during ethanol pretreatment testing. M, ethanol and saccharin deliveries (mean 6 S.E.M.) during saline control testing. From top to bottom, each panel shows increasing pretreatment doses of ethanol. 1997 Ethanol Self-Administration 1255 tration were quickly recovered after the 1.0 and 3 mg/kg doses of CPPene. In fact, on the final day of saline pretreatment before both the 3 and 5.6 mg/kg doses of CPPene, ethanol self-administration was considerably greater than in previous sessions (fig. 5, bottom left). These increases did not, however, appear to be part of a trend, because responding in each of the other three sessions appeared to be stable. Effects of diazepam pretreatment on ethanol and saccharin self-administration. A total of eight animals met the inclusion criteria for the diazepam dose-effect curve determination. The effects of diazepam on session totals of ethanol and saccharin self-administration are shown in figure 6 (top). Only the 5.6 mg/kg dose of diazepam produced a statistically significant [t(7) 5 4.25] decrease in ethanol deliveries. This dose of diazepam also significantly [t(7) 5 5.42] suppressed saccharin responding (fig. 6, top right), resulting in a 71% decrease in saccharin deliveries compared with saline control levels. The daily means of ethanol and saccharin self-administration during diazepam pretreatment are also shown in figure 6 (bottom). The 3 mg/kg dose of diazepam resulted in a gradual decrease in ethanol responding over the final 4 days of treatment (fig. 6, bottom left), but the overall change in ethanol self-administration at this dose was not statistically significant. Saccharin responding during the same period remained stable (fig. 6, bottom right). The 5.6 mg/kg dose of diazepam completely abolished ethanol responding during the first 2 days, with a gradual recovery occurring on the last 2 days of diazepam pretreatment. A similar pattern of modest recovery occurred for saccharin self-administration. Effects of pentobarbital pretreatment on ethanol and saccharin self-administration. A total of 11 rats reached criteria for inclusion in the pentobarbital dose-effect curve. Pentobarbital, at a dose of 3 mg/kg, had no effect on either ethanol (fig. 7, top left) or saccharin (fig. 7, top right) self-administration. However, subsequent baseline levels of ethanol self-administration were lower after this dose of pentobarbital (fig. 7, top left). Saccharin deliveries were not affected in this manner and, in fact, increased somewhat from the first to the second saline control block. The 10 mg/kg dose of pentobarbital differentially affected ethanol and saccharin deliveries, in that only saccharin deliveries were significantly reduced from baseline rates (fig. 7, top right) [t(10) 5 3.375]. Similarly, although the 20 mg/kg dose of pentobarbital somewhat reduced ethanol deliveries, saccharin selfadministration was decreased significantly [t(10) 5 6.56] and to a much greater degree. At a gross observational level, the 20 mg/kg dose of pentobarbital resulted in sedation and loss of righting reflex at the start of the session in the majority of the animals tested. Daily totals of ethanol and saccharin deliveries showed substantial variability over days. This fact was most evident Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 4. Effects of noncontingent PCP pretreatment on ethanol and saccharin self-administration. Top, ethanol (left) and saccharin (right) deliveries after pretreatment with PCP (f) and deliveries during pretest saline control sessions (M). Bottom, left, daily means of ethanol deliveries during noncontingent PCP administration (f) and during pretest saline control sessions (M); right, daily means of saccharin deliveries during noncontingent PCP administration (F) and during pretest saline control sessions (E). Points shown are the number of deliveries (mean 6 S.E.M.) of ethanol and saccharin received in the last four sessions of a six-session block at each condition. *, statistically significant effects (P , .05). 1256 Shelton and Balster Vol. 280 at the 20 mg/kg dose of pentobarbital, at which ethanol self-administration on day 2 (day 4 of testing) was very low, compared with the other 3 days at this dose (fig. 7, bottom left). Saccharin deliveries, at doses of 3.0 and 10.0 mg/kg pentobarbital, showed a gradual decrease over days (fig. 7, bottom right), not unlike that seen for ethanol self-administration after low doses of noncontingent ethanol. Discussion This examination of the effects of GABA agonists and NMDA antagonists on a multiple schedule of ethanol and saccharin self-administration resulted in a number of findings. Clearly, the study showed that it is possible to train rats to self-administer ethanol and saccharin under a multiple schedule. Moreover, it was shown that ethanol could serve as a reinforcer, relative to water, in the absence of any experimental manipulations other than food restriction. Responding for ethanol was robust, and a clear separation between responding for ethanol and water quickly developed over the course of the 10 test sessions. This crucial demonstration of ethanol reinforcement, relative to vehicle, is implicit in most operant ethanol self-administration studies but is rarely closely examined or emphasized. Pretreament of the rats with ethanol produced differential, dose-dependent effects on ethanol and saccharin self-administration. The 1000 mg/kg pretreatment dose of ethanol suppressed both ethanol and saccharin self-administration, although ethanol was decreased to a much greater degree than was saccharin. The 1560 mg/kg dose of ethanol selectively and significantly suppressed ethanol self-administration relative to saccharin. At this dose, ethanol deliveries were greatly decreased, whereas saccharin deliveries were not altered. The results are complicated by the fact that saccharin self-administration increased during both the ethanol and saline pretreatment sessions and continued to increase during determination of the ethanol pretreatment dose-effect curve. Based on this continuous increase, it is likely that saccharin self-administration had not reached a stable level before the beginning of the ethanol pretreatment curve. Nevertheless, the possibility that ethanol injections caused the increases in saccharin self-administration cannot be ruled out. There are a number of possible mechanisms through which noncontingent ethanol might selectively reduce ethanol intake. One possibility relates to food restriction. It is possible that noncontingent ethanol pretreatment replaced the calories provided by self-administered ethanol and thereby reduced ethanol self-administration. This hypothesis is un- Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 5. Effects of noncontingent CPPene pretreatment on ethanol and saccharin self-administration. Top, ethanol (left) and saccharin (right) deliveries after pretreatment with CPPene (f) and deliveries during pretest saline control sessions (M). Bottom, left, daily means of ethanol deliveries during noncontingent CPPene administration (f) and during pretest saline control sessions (M); right, daily means of saccharin deliveries during noncontingent CPPene administration (F) and during pretest saline control sessions (E). Points shown are the number of deliveries (mean 6 S.E.M.) of ethanol and saccharin received in the last four sessions of a six-session block at each condition. *, statistically significant effects (P , .05). 1997 Ethanol Self-Administration 1257 likely for a number of reasons. Firstly, self-administration of other drugs of abuse that have no caloric or anorectic properties is increased by food deprivation (Macenski and Meisch, 1994; Meisch, 1987). Although not conclusive proof, the fact that the self-administration of other drugs is also affected in this manner does suggest that the rats were not drinking ethanol primarily for its caloric value. In addition, ethanol self-administration increased during acquisition even though the level of food restriction remained constant. Finally, the finding that ethanol drinking decreased when presession feeding was discontinued, rather than increased because of this loss of presession food, also supports the contention that caloric restriction increased ethanol drinking by enhancing the reinforcing properties of ethanol. A second possible explanation for the selective effect of ethanol on ethanol self-administration is that ethanol pretreatment produced effects similar to those of self-administered ethanol, thereby reducing the reinforcing effects of ethanol. This conclusion is necessarily tentative, but it is supported by two other studies using different species, routes of ethanol administration and experimental methodologies (Karoly et al., 1978; Petry, 1995). It is interesting to note that ethanol is the only drug that has been shown to produce this effect (Karoly et al., 1978; Petry, 1995). For example, noncon- tingent cocaine does not suppress cocaine self-administration (Skjoldager et al., 1993), dizocilpine is not effective in selectively reducing oral PCP self-administration (Carroll et al., 1994) and methadone does not selectively attenuate alfentanil self-administration (Mello et al., 1983) . Neither the noncompetitive NMDA antagonist PCP nor the competitive NMDA antagonist CPPene selectively suppressed ethanol self-administration, compared with saccharin self-administration. The inability of PCP or CPPene to selectively decrease ethanol self-administration is in contrast to other literature reports. Only two other studies have directly examined the effects of NMDA antagonists on ethanol self-administration. Both of those studies found that intraaccumbens injections of the competitive NMDA antagonist AP-5 decreased ethanol self-administration, compared with water (Rassnick et al., 1992a,b). There are a number of methodological differences between these experiments that may account for the different findings. Firstly, the cited studies injected AP-5 directly into the nucleus accumbens, rather than systemically. A second relevant difference between the present study and past experiments is the use of a saccharin baseline rather than a water baseline. Water responding is typically very low in water-satiated animals and is therefore Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 6. Effects of noncontingent diazepam pretreatment on ethanol and saccharin self-administration. Top, ethanol (left) and saccharin (right) deliveries after pretreatment with diazepam (f) and deliveries during pretest saline control sessions (M). Bottom, left, daily means of ethanol deliveries during noncontingent diazepam administration (f) and during pretest saline control sessions (M); right, daily means of saccharin deliveries during noncontingent diazepam administration (F) and during pretest saline control sessions (E). Points shown are the number of deliveries (mean 6 S.E.M.) of ethanol and saccharin received in the last four sessions of a six-session block at each condition. *, statistically significant effects (P , .05). 1258 Shelton and Balster Vol. 280 probably not as sensitive to drug-induced rate decreases as the saccharin baseline in the present studies. As was the case with NMDA antagonists, pretreatment of rats with the indirectly acting GABAa agonists diazepam and pentobarbital also failed to selectively suppress ethanol selfadministration relative to saccharin responding. Other laboratories have shown both increases (Barrett and Weinberg, 1975; Petry, 1995) and decreases (Chan et al., 1983a,b; Roehrs et al., 1984; Samson and Grant, 1985) in ethanol selfadministration after benzodiazepine administration. Direct comparison of our findings and those in previous studies is difficult because of the different methodologies. Among the literature reports, only three studies have used operant techniques and, of these, only two have examined the specificity of benzodiazepines for decreasing ethanol self-administration relative to another reinforcer (Petry, 1995; Samson and Grant, 1985). However, there are a number of possible reasons for the divergence in findings between our study and previous investigations. One possibility is that there are differences among benzodiazepines in their ability to selectively reduce ethanol self-administration. Another difference is in the choice of reporting measures used. We reported actual numbers of ethanol and saccharin deliveries, whereas the other studies used either percentages of baseline (Samson et al., 1982) or responses per second under a variable-interval 5-sec schedule, in which decreases in responding may not directly affect rates of reinforcement (Petry, 1995). Overall, the general lack of specificity for both NMDA antagonists and GABA agonists reported here could have been the result of a number of factors. It has been shown that there is a great deal of uniformity of drug effects on operant behavior, regardless of the reinforcer used to maintain that behavior. For instance, d-amphetamine can increase both food- and shock-maintained behavior when administered under proper conditions (Barrett, 1977), even though the two maintaining events are radically different. Therefore, it is not surprising that the majority of the pretreatment drugs in the present study produced nonselective effects. If all maintaining events (drug or another reinforcer) are fundamentally similar, it may be difficult to infer specific neurochemical processes based on self-administration data. It is also possible that selective decreases in ethanol self-administration were masked by the combined pharmacological and behavioral effects of pretreatment and self-administered drugs. This hypothesis could partially account for the fact that the pretreatment drugs decreased saccharin self-administration more than ethanol self-administration. It is conceivable that the pretreatment injection was additive with early-session ethanol self-administration and thereby produced greater effects on saccharin responding that occurred later in the Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 Fig. 7. Effects of noncontingent pentobarbital pretreatment on ethanol and saccharin self-administration. Top, ethanol (left) and saccharin (right) deliveries after pretreatment with pentobarbital (f) and deliveries during pretest saline control sessions (M). Bottom, left, daily means of ethanol deliveries during noncontingent pentobarbital administration (f) and during pretest saline control sessions (M); right, daily means of saccharin deliveries during noncontingent pentobarbital administration (F) and during pretest saline control sessions (E). Points shown are the number of deliveries (mean 6 S.E.M.) of ethanol and saccharin received in the last four sessions of a six-session block at each condition. *, statistically significant effects (P , .05). 1997 References AIGNER, T. G. AND BALSTER, R. L.: Rapid substitution procedure for intravenous drug self-administration studies in rhesus monkeys. Pharmacol. Biochem. Behav. 10: 105–112, 1979. BARRETT, J. E.: Behavioral history as a determinant of the effects of damphetamine on punished behavior. Science (Wash.. DC) 198: 67–69, 1977. BARRETT, J. E. AND WEINBERG, E. S.: Effects of chlordiazepoxide on scheduleinduced water and alcohol consumption in the squirrel monkey. Psychopharmacologia 40: 319–328, 1975. CARROLL, M. E., CARMONA, G. N. AND RODEFER, J. S.: Phencyclidine (PCP) self-administration and withdrawal in rhesus monkeys: Effects of buprenorphine and dizocilpine (MK-801) pretreatment. Pharmacol. Biochem. Behav. 48: 723–732, 1994. CHAN, A. W. K., LEONG, F. W. AND SCHANLEY, D. L.: Influence of chlordiazepoxide on alcohol consumption in mice. Pharmacol. Biochem. Behav. 18: 797–802, 1983a. 1259 CHAN, A. W. K., SCHANLEY, D. L. AND LEONG, F. W.: Long-lasting reduction in ethanol selection after involuntary intake of ethanol/chlordiazepoxide. Pharmacol. Biochem. Behav. 19: 275–280, 1983b. COOPER, B. R., VIIK, K., FERRIS, R. M. AND WHITE, H. L.: Antagonism of the enhanced susceptibility to audiogenic seizures during alcohol withdrawal in the rat by g-aminobutyric acid (GABA) and GABA-mimetic agents. J. Pharmacol. Exp. Ther. 209: 396–404, 1979. DANIELL, L. C.: The noncompetitive N-methyl-D-aspartate antagonists, MK801, phencyclidine and ketamine, increase the potency of general anesthetics. Pharmacol. Biochem. Behav. 36: 111–115, 1990. DAOUST, M., SALIGAUT, C., LHUINTRE, J. P., MOORE, N., FLIPO, J. L. AND BOISMARE, F.: GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol 4: 469–472, 1987. FADDA, F., ARGIOLAS, A., MELIS, M. R., DE MONTIS, G. AND GESSA, G. L.: Suppression of voluntary ethanol consumption in rats by g-butyrolactone. Life Sci. 32: 1471–1477, 1983. GALLIMBERTI, L., GENTILE, N., CIBIN, M., FADDA, F., CANTON, G., FERRI, M., FERRARA, S. D. AND GESSA, G. L.: g-Hydroxybutyric acid for treatment of alcohol withdrawal syndrome. Lancet 1: 787–789, 1989. GONZALES, R. A. AND WOODWARD, J. J.: Ethanol inhibits N-methyl-d-aspartatestimulated [3H]norepinephrine release from rat cortical slices. J. Pharmacol. Exp. Ther. 253: 1138–1144, 1990. GRANT, K. A.: Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav. Pharmacol. 5: 383–404, 1994. GRANT, K. A. AND COLOMBO, G.: Discriminative stimulus effects of ethanol: Effect of training dose on the substitution of N-methyl-d-aspartate antagonists. J. Pharmacol. Exp. Ther. 264: 1241–1247, 1992. GRANT, K. A., KNISELY, J. S., TABAKOFF, B., BARRETT, J. E. AND BALSTER, R. L.: Ethanol-like discriminative stimulus effects of noncompetitive N-methyl-daspartate antagonists. Behav. Pharmacol. 2: 87–91, 1991. HUBNER, C. B. AND KOOB, G. F.: Bromocriptine produces decreases in cocaine self-administration in the rat. Neuropsychopharmacology 3: 101–108, 1990. JUNE, H. L., COLKER, R. E., DOMANGUE, K. R., PERRY, L. E., HICKS, L. H., JUNE, P. L. AND LEWIS, M. J.: Ethanol self-administration in deprived rats: Effects of Ro15–4513 alone, and in combination with flumazenil (Ro15–1788). Alcohol. Clin. Exp. Res. 16: 11–16, 1992. KAROLY, A. J., WINGER, G., IKOMI, F. AND WOODS, J. H.: The reinforcing property of ethanol in rhesus monkey. Psychopharmacology. 58: 19–25, 1978. KUBENA, R. K. AND BARRY, H. I.: Generalization by rats of alcohol and atropine stimulus characteristics to other drugs. Psychopharmacology. 15: 196–206, 1969. LILIJEQUIST, S. AND ENGEL, J.: Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 78: 71– 75, 1982. LOVENGER, D. M., WHITE, G. AND WEIGHT, F. F.: Ethanol inhibits NMDAactivated ion current in hippocampal neurons. Science (Wash. DC) 243: 1721–1724, 1989. MACENSKI, M. J. AND MEISCH, R. A.: Oral drug reinforcement studies with laboratory animals: Applications and implications for understanding drugreinforced behavior. Curr. Directions Psychol. Sci. 3: 22–27, 1994. MCBRIDE, W. J., MURPHY, J. M., LUMENG, L. AND LI, T. K.: Effects of Ro15–4513, fluoxetine and desipramine on the intake of ethanol, water and food by the alcohol-preferring (P) and nonpreferring (NP) lines of rats. Pharmacol. Biochem. Behav. 30: 1045–1050, 1988. MEHTA, A. K. AND TICKU, J. K.: Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves g-aminobutyric acid-gated chloride channels. J. Pharmacol. Exp. Ther. 246: 558–564, 1988. MEISCH, R. A.: Factors controlling drug reinforced behavior. Pharmacol. Biochem. Behav. 27: 367–371, 1987. MELLO, N. K., BREE, M. P. AND MENDELSON, J. H.: Comparison of buprenorphine and methadone effects on opiate self-administration in primates. J. Pharmacol. Exp. Ther. 225: 378–386, 1983. MELLO, N. K., KAMIEN, J. B., LUKAS, S. E., DRIEZE, J. AND MENDELSON, J. H.: The effects of nalbuphine and butorphanol treatment on cocaine and food selfadministration by rhesus monkeys. Neuropsychopharmacology 8: 45–55, 1993. MORRISETT, R. A., REZVANI, A. H., OVERSTREET, D., JANOWSKY, D. S., WILSON, W. A. AND SWARTZVELDER, H. S.: MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur. J. Pharmacol. 176: 103–105, 1990. NIE, Z. G., MADAMBA, S. G. AND SIGGINS, G. R.: Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J. Pharmacol. Exp. Ther. 271: 1566–1573, 1994. PETRY, N. M.: Ro15–4513 selectively attenuates ethanol, but not sucrose, reinforced responding in a concurrent access procedure: Comparison to other drugs. Psychopharmacology 121: 192–203, 1995. RASSNICK, S., D’AMICO, E., RILEY, E. AND KOOB, G. F.: GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol. Clin. Exp. Res. 17: 124–130, 1993a. RASSNICK, S., D’AMICO, E., RILEY, E., PULVIRENTI, L., ZIEGLGÄNSBERGER, W. AND KOOB, G.: GABA and nucleus accumbens glutamate neurotransmission modulate ethanol self-administration in rats. Ann. N.Y. Acad. Sci. 654: 502–505, 1992a. RASSNICK, S., PULVIRENTI, L. AND KOOB, G.: SDZ-205,152, a novel dopamine receptor agonist, reduces oral ethanol self-administration in rats. Alcohol 10: 127–132, 1993b. Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 session. Such a drug interaction could have a particularly pronounced effect on the outcome of studies using multiple schedules in general, and the present experiment in particular, which used quickly alternating components of short duration. One possible test of this hypothesis would be to evaluate the same drugs used in this study in animals trained to respond for ethanol and saccharin on successive days. The training procedure and multiple-schedule model used in this study was completely unique among the ethanol selfadministration literature. Because this was the case, there was little with which to directly compare our data. As previously noted, there have been a number of studies using multiple schedules of drug- and food-reinforced responding reported in the literature on i.v. self-administration. The findings of these studies are, for the most part, consistent with the present results. For example, one i.v. self-administration study found that neither cocaine nor the dopamine reuptake blocker GBR 12909 selectively decreased cocaine or GBR 12909 self-administration (Skjoldager et al., 1993). Nonselective effects were also noted after pretreatment with D1 and D2 receptor blockers in rhesus monkeys responding for i.v. cocaine and food (Woolverton and Virus, 1989). Similarly, noncontingent methadone pretreatment does not selectively attenuate heroin self-administration without affecting food-reinforced responding (Mello et al., 1983) . The inability of drugs with behavioral activity to decrease ethanol self-administration without effects of their own is not surprising. Clearly, for any agonist-based intervention to be successful, it must be given in sufficient doses to generate or attenuate some of the reinforcing effects of the abused compound it is intended to replace. A case in point would be methadone, which, as previously mentioned, generally fails to have selective effects on opiate self-administration but has proven to be of substantial clinical utility despite, or perhaps more correctly because, it is given to patients at doses that have a variety of opiate-like pharmacological and behavioral effects. Thus, it seems unlikely that a depressant drug would reduce ethanol self-administration without having other behavioral effects. In conclusion, these negative results should not be taken as evidence that GABA or NMDA receptors are not involved in ethanol reinforcement. Rather, it seems likely that neither the GABAergic nor NMDA receptor system alone is able to entirely account for the reinforcing effects of ethanol. This conclusion is supported by other evidence suggesting that many of the actions of ethanol may arise from a combination of its effects at a number of ligand-gated ion channels (Grant, 1994) . Ethanol Self-Administration 1260 Shelton and Balster SANGER, D. J.: Substitution by NMDA antagonists and other drugs in rats trained to discriminate ethanol. Behav. Pharmacol. 4: 523–528, 1993. SHELTON, K. L. AND BALSTER, R. L.: Ethanol drug discrimination in rats: Substitution with GABA agonists and NMDA antagonists. Behav. Pharmacol. 5: 441–450, 1994. SKJOLDAGER, P., WINGER, G. AND WOODS, J. H.: Effects of GBR 12909 and cocaine on cocaine-maintained behavior in rhesus monkeys. Drug Alcohol Depend. 33: 31–39, 1993. STONE, E. J. AND FORNEY, R. B.: The effects of phencyclidine on ethanol and sodium hexobarbital in mice. Toxicol. Appl. Pharmacol. 40: 177–183, 1977. TAKADA, R., SAITO, K., MATUSRA, H. AND INOKI, R.: Effect of ethanol on hippocampal receptors in the rat brain. Alcohol 6: 115–119, 1989. WESSINGER, W. E. AND BALSTER, R. L.: Interaction between phencyclidine and central nervous system depressants evaluated in mice and rats. Pharmacol. Biochem. Behav. 27: 323–332, 1987. WINTER, J. C.: The stimulus properties of morphine and ethanol. Psychopharmacology 44: 209–214, 1975. WOOLVERTON, W. L. AND VIRUS, R. M.: The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol. Biochem. Behav. 32: 691–697, 1989. Send reprint requests to: Robert L. Balster, Ph.D., Department of Pharmacology/Toxicology, Medical College of Virginia/VCU, Box 980310, Richmond, VA 23298-0310. Downloaded from jpet.aspetjournals.org at ASPET Journals on May 6, 2017 RASSNICK, S., PULVIRENTI, L. AND KOOB, G. F.: Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology 109: 92–98, 1992b. ROBLEDO, P., KANEKO, W. AND EHLERS, C. L.: Combined effects of ethanol and MK-801 on locomotor activity in the rat. Pharmacol. Biochem. Behav. 39: 513–516, 1991. ROEHRS, T., YANG, O. AND SAMSON, H.: Chlordiazepoxide’s interaction with ethanol intake in the rat: Relation to ethanol exposure paradigms. Pharmacol. Biochem. Behav. 20: 849–853, 1984. ROSENBERG, H., SMITH, S. AND CHIU, T. H.: Benzodiazepines specific and nonspecific tolerance following chronic flurazepam treatment. Life Sci. 32: 279– 285, 1983. SAMSON, H. H. AND GRANT, K. A.: Chlordiazepoxide effects on ethanol selfadministration: Dependence on concurrent conditions. J. Exp. Anal. Behav. 43: 353–364, 1985. SAMSON, H. H., HARAGUCHI, M., TOLLIVER, G. A. AND SADEGHI, K. G.: Antagonism of ethanol-reinforced behavior by the benzodiazepine inverse agonists Ro15– 4513 and FG 7142: Relation to sucrose reinforcement. Pharmacol. Biochem. Behav. 33: 601–608, 1989. SAMSON, H. H., ROEHRS, T. A. AND TOLLIVER, G. A.: Ethanol reinforced responding in the rat: A concurrent analysis using sucrose as the alternate choice. Pharmacol. Biochem. Behav. 17: 333–339, 1982. SAMSON, H. H., TOLLIVER, G. A., PFEFFER, A. O., SADEGHI, K. G. AND MILLS, F. G.: Oral ethanol reinforcement in the rat: Effect of the partial inverse benzodiazepine agonist Ro15–4513. Pharmacol. Biochem. Behav. 27: 517–519, 1987. Vol. 280