* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BIOL Unit 4 - Biomolecules
Survey
Document related concepts
Transcript
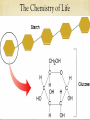
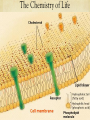
BIOLOGY Biological Molecules The Chemistry of Life Molecules are made of atoms. An atom is the basic unit of all matter. There are three subatomic particles that make up atoms: Protons Neutrons Electrons A chemical element is a pure substance that consists entirely of one type of atom. The main types of chemical bonds are ionic bonds and covalent bonds. The Chemistry of Life The Chemistry of Life Does ice float or sink in liquid water? What does this mean in terms of the density of liquid water versus ice? Cohesion is an attraction between molecules of the same substance. Adhesion is an attraction between molecules of different substances. A mixture is a material composed of two or more elements or compounds that are physically mixed together but not chemically combined. What are some examples of a mixture? What about earth’s atmosphere. Is it a mixture or a pure substance? In a solution of salt water, the salt is the solute and the water is the solvent. The Chemistry of Life A scale that indicates the acidity or basicity of a substance is called the pH scale. It goes from 0 – 14. 0 is highly acidic and 14 is highly basic. Basic solutions have a high concentration of hydroxide ions, OH- and acidic solutions have a high concentration of hydrogen ions, H+. The Chemistry of Life Macromolecules are formed by a process called polymerization. Small particles that make up a larger one are called monomers. The long chain of linked monomers is called a polymer. The Chemistry of Life Four groups of macromolecules found in living things are carbohydrates, lipids, nucleic acids and proteins. https://youtu.be/EtiHCSwVoUc The Chemistry of Life Carbohydrates are compounds made of carbon, hydrogen and oxygem atoms usually in the ratio of 1:2:1. Living things use carbohydrates as their main source of energy. Plants and some animals also use carbohydrates for structural purposes. Living things store extra sugar as complex carbohydrates called starches. Single sugar molecules are called monosaccharides. Large macromolecules formed from many monosaccharides are called polysaccharides. Cellulose is an important plant carbohydrate that makes up cell walls. This is what gives plants rigidity. It is also the major component in wood and paper! The Chemistry of Life The Chemistry of Life The Chemistry of Life Lipids are large and varied. Lipids are generally not soluble in water. Lipids are either fats, oils or waxes. Lipids can be used by living things to store energy. Lipids are important parts of biological membranes and waterproof coverings. Steroids are lipids and serve as chemical messengers. Lipids can be saturated, unsaturated or polyunsaturated. Liquid lipids like olive oil contain unsaturated fatty acids. The Chemistry of Life The Chemistry of Life The Chemistry of Life Proteins are macromolecules that contain nitrogen, carbon, hydrogen and oxygen. Proteins are polymers of amino acids. More than 20 different amino acids are found in nature. Proteins control the rate of some reactions. Proteins regulate cell processes. Proteins are used to form bones and muscles. Proteins transport substances into or out of cells. Proteins help fight diseases. The Chemistry of Life The Chemistry of Life The Chemistry of Life Nucleic acids are macromolecules containing hydrogen, oxygen, nitrogen, carbon and phosphorus. Nucleic acids are polymers of individual monomers called nucleotides. Mono- means single Poly- means many Meros means part Nucleotides consist of a 5 carbon sugar, a phosphate group and a nitrogen base. Nucleic acids store and transmit hereditary, or genetic information. There are two kinds of nucleic acids: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) The Chemistry of Life The Chemistry of Life A chemical reaction is a process that changes one set of chemicals into another. Chemical reactions always involve changes in the chemical bonds that join atoms in compounds. Chemical reactions that release energy occur spontaneously. Chemical reactions that absorb energy do not occur spontaneously without a source of energy. The energy that is needed to get a reaction started is called activation energy. A catalyst is a substance that speeds up the rate of a chemical reaction. Enzymes are proteins that act as biological catalysts and speed up reactions that take place in cells by lowering activation energy. The Chemistry of Life The Chemistry of Life The Chemistry of Life Enzymes work best at certain pH’s. The optimal pH depends on the enzyme. Enzymes also work best at certain temperatures. Most enzymes work best at body temperature. Most cells contain proteins that help turn key enzymes on or off at critical stages in the life of the cell.