* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hydrochlorothiazide - Developing Anaesthesia
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Neuropharmacology wikipedia , lookup
Potassium iodide wikipedia , lookup
Theralizumab wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
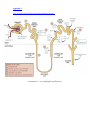
HYDROCHLOROTHIAZIDE Introduction Hydrochlorothiazide is a thiazide diuretic, primarily used in the treatment of hypertension. It may be used alone but more commonly is used in combination formulations with other antihypertensive drugs. Electrolyte disturbances are the most common significant adverse side effect, hypokalemia in particular. History The thiazide diuretics were discovered developed by scientists Karl H. Beyer, James M. Sprague, John E. Baer, and Frederick C. Novello of Merck and Co. in the 1950s. The first drug of this class, chlorothiazide, was marketed under the trade name Duiril in 1958. Chemistry The thiazides are derived from benzothiadiazine, a bicyclic heterocyclic benzene derivative with the heterocycle containing two nitrogens and one sulfur. Classification The principle classes of diuretic agents include: 1. The thiazides: ● Bendroflumethiazide ● Chlorothiazide ● Hydrochlorothiazide ● Chlorthalidone ● Indapamide ● Methyclothiazide ● 2. 3. The loop diuretics: ● Frusemide ● Bumetanide ● Ethacrynic acid The potassium sparing diuretics: ● ● 4. The aldosterone antagonists: ♥ Spironolactone ♥ Eplerenone Direct sodium channel blockers in the distal tubule: ♥ Amiloride. ♥ Triamterene The carbonic anhydrase inhibitors: ● 5. Metolazone Acetozolamide The osmotic diuretics: ● Mannitol Preparation Tablets: ● 25 mg. Fixed dose combinations: There are many fixed dose combinations available including: ● ACE inhibitors: ♥ ● Enalapril, fosinopril, quinapril Sartans: ♥ ● Potassium sparing diuretics: ♥ ● Candesartan, eprosartan, irbesartan, olmesartan, telmisartan, valsartan Amiloride, triamterene. Calcium channel blocking agents: ♥ Amlodipine Mechanism of Action The thiazide diuretics are moderately potent diuretics They inhibit reabsorption of sodium and chloride in approximately equivalent amounts) in the proximal (diluting) segment of the distal convoluted tubule - hence water is retained in the tubule and so lost from the body. Natriuresis may be also be accompanied by some loss of potassium and magnesium. See also Appendix 1 below. Hydrochlorothiazide has properties qualitatively similar to chlorothiazide, although it is about 10 times as potent, on a weight basis When used in recommended low doses for hypertension, the thiazides lower blood pressure principally via a vasodilator effect. 2 Pharmacokinetics Absorption: ● Hydrochlorothiazide is administered orally. Distribution: ● Thiazides can cross the placental barrier. ● Hydrochlorothiazide crosses the placental barrier ● It is excreted in breast milk. ● It does not cross not the blood brain barrier. Metabolism and excretion: ● Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. Pharmacodynamics Onset of the diuretic action following oral administration occurs in 2 hours. Peak diuretic action occurs in about 4 hours. Diuretic activity lasts about 6 - 12 hours. Indications These include: 2 1. Hypertension ● 2. May be used alone but more commonly is used in combination formulations with other antihypertensive drugs. Oedema: Associated with: 3. ● Heart failure ● Hepatic cirrhosis ● Nephrogenic diabetes insipidus Prevention of renal calculi associated with hypercalciuria Contra-indications/precautions These include: 2 1. Hypersensitivity 2. Anuria (contraindicated) 3. Gout: ● 4. May be aggravated by diuretic-induced hyperuricaemia (more likely with higher doses). Diabetics: ● Impaired glucose tolerance - thiazides may increase blood glucose concentration (unlikely to be clinically important at lower doses). 5. Heart failure with significant oedema: ● 6. Conditions or drugs that may cause hypokalaemia: ● 7. Further increases risk of renal impairment and hypotension (particularly in patients with heart failure); monitor renal function and blood pressure (sitting and standing). Hepatic failure: ● 9. Further increases the risk of hypokalaemia; (monitor potassium concentration closely). Conditions or drugs that cause volume depletion: ● 8. Hyponatraemia may occur, particularly if higher doses are used with a salt-restricted diet and/or potassium-sparing diuretics and excess water intake. In cirrhosis, diuretic - induced volume depletion and/or electrolyte disturbance may precipitate hepatic coma or encephalopathy. Elderly: ● Use a lower starting dose; more susceptible to electrolyte imbalance (e.g. hyponatraemia, hypokalaemia) and orthostatic hypotension. Pregnancy Hydrochlorothiazide is a category C drug with respect to pregnancy Category Class drugs are those drugs which, owing to their pharmacological effects, have caused or may be suspected of causing harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible. Specialised texts should be consulted for further details Limited information is available on the use of hydrochlorothiazide during pregnancy. Theoretically, diuretics have the potential to decrease plasma volume, cardiac output and uteroplacental perfusion. However, no congenital malformations have been reported. Thiazide diuretics have been associated with an increased risk of maternal nausea and vomiting, neonatal thrombocytopenia and electrolyte disturbances. 4 Consider an alternative medicine, with more safety information, in women who are planning to become pregnant and during pregnancy. If hydrochlorothiazide is the medicine of choice, monitor both maternal and fetal wellbeing. 4 Breast feeding: Compatible; may suppress lactation Adverse Effects 1. Allergic/ hypersensitivity reactions 2. Electrolyte disturbances: Electrolyte retention: ● Hypercalcaemia: ♥ Urinary calcium excretion may be decreased. Electrolyte loss: Effects on electrolyte loss are dose-dependent, and include:: ● Hyponatraemia: ♥ ● Hydrochlorothiazide induced hyponatraemia is usually only mild and asymptomatic. Hypokalaemia: ♥ Reduce the risk by using a low dose. Hypokalaemia is less likely when also taking an ACE inhibitor, sartan or potassium-sparing diuretic 3. ● Hypomagnesaemia ● Hypochloraemic alkalosis Volume depletion: ● Especially in association with other drugs or conditions that lead to hypovolemia. Postural hypotension may the first sign. 4. Hyperuricaemia: ● 5. Thiazides impair urinary calcium excretion Hyperglycaemia: ● Thiazide therapy may impair glucose tolerance. Dosing Most adverse effects are dose-related; start with a low dose and increase slowly. Hypertension: ● 12.5 - 50 mg daily. Oedema: ● 2 2 25 - 100 mg daily, or intermittently on 3 - 5 days each week. The maximum recommended daily dosage is 200 mg. 3 Thiazide diuretic induced hypokalaemia: For thiazide diuretic induced hypokalaemia: 1. Risk can reduced by using the lowest effective dose 2. Is less likely when also taking an ACE inhibitor, sartan or potassium-sparing diuretic 3. Potassium supplements may be used to treat mild hypokalaemia: ● Each potassium chloride 600 mg tablet contains 8 mmol potassium; daily potassium replacement requirement is around 20 - 60 mmol (3 - 8 tablets). Appendix 1 Sites of action of the 5 principle groups of diuretic agents: (Cassandra Uy - www.pathophys.org/diuretics/) References 1. eTG - November 2015. 2. Hydrochlorothiazide in Australian Medicines Handbook, Website Accessed June 2015. 3. Hydrochlorothiazide in MIMs on Line - 1 October 2013 4. RWH Clinical Guidelines - Hypertension in Pregnancy, 27 February 2015.