* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drug Development and Assessment in Man Pharmaceutical Medicine
Development of analogs of thalidomide wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Clinical trial wikipedia , lookup
Compounding wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
List of off-label promotion pharmaceutical settlements wikipedia , lookup
Drug design wikipedia , lookup
Patent medicine wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacognosy wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacovigilance wikipedia , lookup
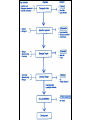
Drug Development and Assessment in Man Pharmaceutical Medicine Thursday 11th October 2007 Dr John Stinson An expert is somebody who is more than 50 miles from home, has no responsibility for implementing the advice he gives, and shows slides. Edwin Meese What do Doctors Do? • • • • • • • Diagnose and treat Cost of diagnosing What do we cure? What do we alleviate? How do we achieve these effects? Who makes medicines? Future threats? Antibiotic Resistance • • • • • • • • • 1937 1944 1947 1947 1947 1952 1953 1956 1960 Sulphonamides Penicillin Cephalosporins Chloramphenicol Aminoglycosides Macrolides Tetracyclines Glycopeptides Flucloxacillin • • • • • 1961 Rifampicin 1962 Fusidic Acid 1970 Trimethoprim 1975 Carbipenems 1982 Fluoroquinolones • A new class of antibiotic every 3 years • 18 year gap to 2000 Introduction • • • • • • • 1935 <1950 1950s 1960s 1970s 1980s 1990s Few effective Medicines No antihypertensive agents Diuretics B2 Receptor antagonists Calcium Channel antagonists ACE Inhibitors Angiotensin II receptor antagonist • BNF 2004 > 100 antihypertensives • Before 1960 Random screening and empirical drug design i.e. LUCK! • 1960s Medicinal Chemistry Better organic Biochemistry Mass spectroscopy NMR developments • 1970s Receptor Science Agonists/Antagonists • 1980s Protein Chemistry Enzyme Chemistry Inhibitors of Enzymes • 1990s Molecular Biology Gene Therapy Biopharmacuticals Receptor Science Discovered Medicines Receptor Agonist/Antagonist Disease Benzodiazepine Diazepam Opiate B2 B1 & B2 Morphine Salbutamol Propanolol Epilepsy, Anxiety Sedation Analgesia Asthma Hypertension H2 Dopamine 5HT AII Cimetidine Levodopa Ondansetron Losartan Peptic Ulceration Parkinsonism Emesis Hypertension Non Peptide Enzyme Inhibitors Enzyme Inhibitor Therapy ACE HMG CoA DHFR b-lactamase 14 a lactamase Captopril Simvastatin Trimethoprim Clavulinic acid Ketoconazole Hypertension Hi Cholesterol Antibacterial Antibiotic Adjunc Antifungal Molecules Registered in Libraries • • • • • • Screened in biological systems >100,000 molecules screened Automated systems Intelligent screening using 3-D structures Molecule Receptor Binding Appropriate shaped molecule tried Potential Drug Candidate • • • • • More intensely assessed for activity As it passes more hurdles Proceed into toxicology testing Now considered a New Chemical Entity NCE New Chemical Entity • What route of administration? Parenteral Oral Transcutaneous Subcutaneous Inhalation Rectal Eye Buccal NCE Formulation • • • • • • • • Tablet Suspension Solution Capsule Enteric coated Cream Ointment Pro-drug? Route of Synthesis? • Economics (platinum) • Which Salt? Hydrocortisone = mild steroid Hydrocortisone butyrate = potent • Solubility • Physicochemical properties • Stability • Compatibility with excipients Clinical Pharmacology • The Scientific basis of drug therapy, includes: Pharmacokinetics Pharmacodynamics Pharmaceutical development Pharmacovigilance Pharmacoeconomics Pharmacoepidemiology Pharmaceutical Process • Is the drug getting into the patient? Route? Formulation? Dissolution? Absorption? Pharmacokinetic Process • Is the drug getting to its site of action? Absorption? Distribution? Metabolism? Excretion? Pharmacodynamic & Therapeutic Process Is the drug producing the required pharmacological effect? Is the pharmacological effect being translated into a therapeutic effect? Phase 1 Trials • Initial studies in man to determine tolerance and the safe dosage range and to give indication of metabolic handling. These studies are usually undertaken with healthy volunteers but may be extended to include patients. Pharmacokinetic (ADME) and pharmacodynamic activities are measured if possible. • N= 30-80 Phase II Trials • Early controlled trials in a limited number of patients (with the disease) under closely monitored conditions to show efficacy and short term safety. • Humans exposed 250-500 Phase III Trials • Extended large-scale trials to obtain additional evidence of efficacy and safety, and definition of most common adverse effects. Longer term trials possible • Humans exposed 300 - 10,000+ Phase IV Trials • These are performed after the medicine has been licensed and marketed. Postmarketing surveillance occurs after the clinical trials programme is complete. It is used to collect adverse event data from a large patient population. • Humans exposed 10,000+ When Phase I to III Complete • • • • • Apply for Product Licence or authorisation From FDA From EMEA From National authority (IMB etc) Decision based on Safety Data Efficacy Data Pharmaceutical Quality Pharmacovigilance • • • • • Sulfanilamide one of first antibiotics Effective against streptococcal infections Not under patent protection (1908) Many manufacturers marketed it A small company decided to produce a liquid formulation • Found that diethylene glycol was a suitable solvent Pharmacovigilance • Raspberry tasting elixir of sulfanilamide • 72% diethylene glycol, 16% water, 10% sulfanilamide • “Control laboratory” found it suitable with regards to appearance, flavour and flagrance. • There was no toxicity testing of the ingredients Pharmacovigilance • 105 patients died (out of 353 treated) • A mass poisoning • The only rule broken by the manufacturer, the Massengill Company, was that it called the product an elixir although it did not contain ethanol! • The FDA changed the law: • Manufacturers had to prove safety before marketing a medicine. Toxicology Testing • Thalidomide 1956 Germany Antiemetic in pregnancy • 1961 Reports of Phocomelia no cases in 1949-1959 477 cases in 1961 alone 400-500 cases in UK 1959-61 • 1963 CSM in UK • 1968 Medicines Act UK Regulatory Control Pharmacovigilance • • • • • Thalidomide was not approved in US However studies were undertaken in US 624 Pregnant women received thalidomide 10 cases of Phocomelia occurred FDA tightened rules to all stages of drug development • This required extensive testing in animals first Before NCE tested in Man • Safety Pharmacology in Animals Dog&Rat CNS Activity CVS Activity Respiratory Activity • Pharmacokinetics Dog & Rat usually Absorption Distribution Metabolism Excretion Before NCE tested in Man • Acute Toxicity single dose 2 Species of animlas by 2 routes Usually IV and Oral (or proposed route) Maximum well tolerated dose • Repeat dose toxicity Rodent and non-rodent Using route proposed for man Duration depends on proposed duration in man Before NCE tested in Man • Genotoxicity Ames Test- bacteria gene mutation S.Typhi, E.Coli Mouse Lymphoma Cell line – mammalian gene mutation Chinese Hamster Ovary – chromosomal damage Micronucleus Test mammalian in vitro chromosome damage Assay of DNA synthesis in rat liver • Reproductive toxicity only if women of child bearing potential Adverse Drug Reaction Reporting Pharmacovigilance • When a new medicine gets a licence • On average about 4,000 humans have received it in trials • Many have only received it for a short time • If an adverse event only occurs in 1: 5,000 • No chance to detect it • Especially if it occurs rarely in background • Pharmacovigilance only starts with licence VIOXX –Rofecoxib • • • • • MSD – COX 2 inhibitor In theory less risk of GI bleeds Approved by FDA in 1999 $ 2,500,000,000 per year VIGOR study RR 5 x of AMI compared to naproxen • FDA estimate 27,000 excess AMI/deaths between 1999-2003 VIOXX • • • • • APPROVe study Study in preventing colonic polyps Showed increased CV deaths September 30th 2004 product withdrawn Cost will probably be $9 billion in sales & $5 billion in law suits Bayer statin • Statin withdrawn due to rhabdomyolisis • Dose response curve not properly explored • Could have been avoided? • Pharmacovigilance does not stop at licensing, indeed it really only starts then Pharmacovigilance Methods • Spontaneous Reporting • Cohort Studies Defined size group of patients Followed for defined period of time 10,000 pts recruited from 2500 GPs Non-promotional, only if going to be Rx Doctor reports and ADEs Can come up with new ADEs “Hypotheses generating” Pharmacovigilance Methods • Case Control studies Hypothesis testing, not generating Select cohort with suspected disease/ADE Select larger cohort without ADE Look for differences in exposure to drug Pharmacovigilance Methods • Computerised databases Prescriptions/Patients Linked To medical adverse events/disease Getting better as I.T. improves Depends on quality of data entered The interface between the medical profession and the pharmaceutical industry • Research State funded = €30 million/year • Research Funded by Pharma = €50 million • Approx 160 doctors, nurses and scientists are funded by pharma industry • Used to be area of growth --now ? • Fraud rare but does occur Medicine Promotion • Advertisements Strict Guidelines safe, best, most etc. reminder vs full advertisement Code of practice Complaints procedure Medicine Promotion • Representatives Educational Informative Promotional Not paid by commission Rising standards Must have data sheet Must report ADEs Must not mislead Medicine Promotion • • • • S T E P safety tolerability efficacy price Medicine Promotion • Samples • • • • must be in response to signed dated request No more than 6 samples per year Smallest pack available Marked “not for resale/free medical sample” Medicine Promotion • Sponsorship IPHA code of practice (new 6th edition) Doctors should not ask for a fee for apt Sponsorship should be appropriate & not out of proportion Sponsored meetings must have major educational component No sponsorship for non-medical people