* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Glycerol + Fatty acids
Epoxyeicosatrienoic acid wikipedia , lookup
Low-density lipoprotein wikipedia , lookup
Cholesterol wikipedia , lookup
High-density lipoprotein wikipedia , lookup
Ethanol-induced non-lamellar phases in phospholipids wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Phospholipid-derived fatty acids wikipedia , lookup
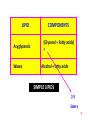
BCM 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 1 -2011/12) 1 LIPID Learning outcome (Objectives) ● Function and distribution. ● Characteristics of fatty acids-structure and chemical properties. ● Saturated and unsaturated fatty acids . ● Structures and properties of phospholipids, sphingolipids, waxes, terpenes and steroids. 2 3 4 5 ??????? 6 LIPID DEFINITION : General definition – all compounds called fat and oils TECHNICAL DEFINITION Fat : Triglycerides in the form of solids at room temperature Oils : Triglycerides which are liquid at room temperature 7 General Definition Any natural compound which is insoluble or nearly insoluble in water but soluble in nonpolar solvents – a. Chloroform b. CS2 c. Ether d. warm or e. hot ethanol 8 FUNCTIONS Lipids are widely distributed in both animal and plant systems and perform a wide variety of functions i. Structural functions - Components of membranes ii. Storage forms of carbon and energy iii. precursor for major compounds – e.g. hormones. iv. Insulators - thermal, electrical or physical shock v. protective coatings – prevent infections, loss or addition of compounds vi. Regulators - as vitamins & hormones 9 CLASSIFICATION 1. SIMPLE LIPIDS Fatty acid esters (Acid + alcohol ester) 2. COMPPOUND LIPID Fatty acid + alcohol + OTHER COMPOUNDS 10 LIPID Acyglycerols Waxes COMPONENTS (Glycerol + Fatty acids) = Alcohol + fatty acids SIMPLE LIPIDS ??? Esters 11 COMPOUND LIPIDS 4 types of Compound lipid i. Phosphoglycerides ii. Sphingolipids iii. Cerebrosides iv. Gangliosides 12 LIPID COMPONENTS i Phosphoglycerides Glycerol + Fatty acid +HPO42- + satu OHR ii Sphingolipids Sphingosine + Fatty acid + HPO42- + Choline Cerebrosides Sphingosine +Fatty acid + Simple sugar Gangliosides Sphingosine + Fatty acid+ 26 Simple sugar (Including sialic acid) iii iv COMPOUND LIPID 13 i & ii = Phospholipid - presence of phosphate ii , iii & iv = Sphingolipids - presence of Sphingosine iii & iv glycolipid - presence of carbohydrate = 14 GLYCEROL – Trihydroxy alcohol 15 16 FATTY ACIDS ● Long chain aliphatic carboxylic acids- contains carboxyl group – polar head and `tail’ containing hydrocarbon chain ● Amphiphilic compounds – hydrophilic head and hydrophobic tail ● COOH can be ionised ● Monocarboxyilic acids – linear hydrocarbon chain, even carbon numbers – between C12-C20 ● Short, longer , branched, cyclic and odd numbers also exist BUT not many 17 Octadenic acid 18 FATTY ACIDS 2 TYPES 1. Saturated Fatty acids 2. Unsaturated Fatty acids 19 Structure of Fatty Acids - Saturated Fats ● mostly from animal sources, ● have all single bonds between the carbons in their fatty acid tails, thus all the carbons are also bonded to the maximum number of hydrogens possible. ● saturated fats ● The hydrocarbon chains in these fatty acids are, thus, fairly straight and can pack closely together, making these fats solid at room temperature. 20 Saturated fatty acid –e.g. 1. palmitic acid (CH3(CH2)14COOH) (16C) & 2. Stearic acid (CH3(CH2)16COOH) 21 Saturated Fatty Acids 22 Structure of Fatty Acids - Unsaturated ● Unsaturation normally at - C18 & C20 – double bond separated by methylene group -CH = CH - CH2 - CH = CH ● Double bonds = cis configuration ● Unsaturated fatty acid - oleic (18:1), Linoleic (18:2), Linolenic (18:3) & arachidonic (18:4) 23 Unsaturated fatty acids ● C=C double bond arranged in two ways ● In cis bonds, the two pieces of the carbon chain on either side of the double bond are either both “up” or both “down,” such that both are on the same side of the molecule ● In trans bonds, the two pieces of the molecule are on opposite sides of the double bond, that is, one “up” and one “down” across from each other ● Naturally-occurring unsaturated vegetable oils have almost all cis bonds, but using oil for frying causes some of the cis bonds to convert to trans bonds 24 TRANS CIS 25 26 Unsaturated Fatty Acids 27 ● fatty acids with trans bonds are carcinogenic, or cancer-causing. ● containing products such as margarine are quite high, 28 Oils ● ● ● ● ● mostly from plant sources, have some double bonds between some of the carbons in the hydrocarbon tail, causing bends or “kinks” in the shape of the molecules. Because some of the carbons share double bonds, they’re not bonded to as many hydrogens oils are called unsaturated fats. kinks unsaturated fats can’t pack as closely together, making them liquid at room temperature 29 CIS TRANS 30 31 Making margarine ● Vegetable oils often contain high proportions of polyunsaturated and mono-unsaturated fats (oils) liquids at room temperature. ● You can "harden" (raise the melting point of) the oil by hydrogenating it in the presence of a nickel catalyst. 32 33 34 SIMPLE LIPIDS 2 GROUPS i. Neutral acyglycerols (e.g. Triacylglycerol) ii. Waxes Acyglycerols = glyceride = a tryhydroxy alcohol ester = glycerol + fatty acid (3 different fatty acids) = can be esterified 35 Glycerol = trihydroxy alcohol 36 TRIACYGLYCEROLS 37 38 39 40 41 ● Triacylglycerol – the most abundant ● No ionic groups - neutral lipids ● Triacylglycerol = neutral fats (solids) @ neutral oils (liquid) 42 FUNCTIONS IN ANIMALS I. Adipose tissues - `fat depots' = storage forms of carbon and energy II. Transport - chylomicrons - = lipoprotein – fatty acids are transported through lymphatic system and blood tissue adipose tissues and other organs III. `Physical protection' - e.g. temperature. 43 44 45 46 WAXES ● Also an ester - alcohol & fatty acid = very long hydrocarbon chain – commercial application ● hairs, skin, leaves, fruits 47 WAXES Asid Oleic 48 CHEMICAL CHARACTERISTICS OF TRYACYLGLYCEROL (Reactions of Triacylglycerol) 1. Hydrogenation 49 Double bonds in vegetable oils can be hydrogenated oils become solids – can control - e.g.. peanut butter - crunchy, creamy HYDROGENATION PROCESS 50 2. Halogenation – Addition of halogens Other halides - Iodine(I2), Chlorides (Cl2) 51 ● Saturated fatty acid – iodine number = 0 ● Oleic acid - 90, ● linoleic- 181, ● Linolenic = 274 52 ● Animal fat-iodine number is low ● Vegetable oils – iodine number is high 53 54 3. Hydrolysis (i.) Base Hydrolysis Fatty acid + Glycerol or Salts of fatty acid + Glycerol ● inside cells – by enzymes (lipase) – very specific for ester bonds – products are glycerol + fatty acids ● Non-enzymatic- with alkali (base) salts of fatty acid + Glycerol ● salts of fatty acids = soap Base Hydrolysis = SAPONIFICATION 55 SAPONIFICATION ● The reaction of triacylglycerol with base (alkali) e.g.. NaOH, KOH ● Triacylglycerol – presence of strong ester bond ● Ester bond can be hydrolyzed by base salts of fatty acid + glycerol Salts = soap – react as a soap/detergent 56 Saponification reaction (Base Hydrolysis) 57 If R= palmitic acid Sodium palmitate If R’= oleic acid Sodium oleate R”= stearic acid Sodium stearate 58 59 Detergent? =`surface active agents' – lower surface tension of surface of water H2O = `poor cleansing agent - Y? Because the molecule is very polar and tend to stick to each other – therefore cannot enter non-polar areas like grease, oil, dirt 60 HOW DOES A DETERGENT WORK ?? i. Hydrophobic tails enters grease layers ii. Hydrophilic heads come into contact with aqueous layer separate grease layer from the surface iii. Small grease globules form- `pincushion‘ iv. These globules have similar charges - therefore cannot go near each other – can wash 61 Water Grease 62 63 64 Head Polar (hidrofilik) EkorTak polar (Hidrofobik) 66 (ii). Acid Hydrolysis Carboxylic acids 67 4. RANCIDITY Expose triacylglycerol to warm and moist air rancid (tengik) 2 reactions take place 1. Ester hydrolysis 2. Oxidation of the double bonds ● Hydrolysis - water (inside the lipid) + enzyme (bacteria in the air) ● Oxidation-by O2 on the side chain of triacylglycerol short chain fatty acids – rancid (tengik) 68 69 Phosphoglycerides Phosphoglycerides = Phosphoglycerol i.e. they are derived from glycerol Fatty acids Glycerol Phosphate group 70 Glycerol @ other alcohols Phosphoglycerol = Phosphoglyceride 71 Glycerol (Trihydroxyglycerol) Phosphatidic acid (Glycerol + 2 fatty acids + Phosphate) Phosphoglyceride (Phosphoglycerol) (Glycerol + 2 fatty acids + Phosphate + other group e.g.. alcohol) 72 All phosphoglycerides are Phospholipids!!!! 73 Phosphoglycerides can be further esterified to form other lipids i. Phosphatidylcholine ( choline ester) ii. Phosphatidylethanolamine (ethanolamine) iii. Phosphatidylserine (serine) All are important components of membranes 74 Asid lemak Phosphate 75 Phosphatidylethanolamine 76 Phosphatidylethanolamine 77 Phosphatidylserine 78 Membrane 79 SPHINGOLIPID ● No glycerol – replaced with amine alcohol = Sphingosine ● Number of carbon atoms –varies ● The simplest = ceramides = Fatty acid + sphingosine through amino group via amide bond ● Sphingomyelin – an example of sphingolipid - 1o alcohol esterified to phosphate amino alcohol (= choline) Found in nerve membranes and brain 80 SPHINGOLIPID 1. What is the main structure for sphingolipid? Sphingosine 2. Draw the structure of sphingosine 3. Draw the structure of glycerol and compare between the two CHCH(CH2)12CH3 CHOH Sphingosine CH NH2 CH2OH H2C OH H2COH H2C OH Glycerol 81 SPHINGOLIPID ● No glycerol – replaced with amine alcohol = Sphingosine ● ● Number of carbon atoms –varies The simplest = ceramides = Fatty acid + sphingosine through amino group via amide bond Sphingomyelin – an example of sphingolipid - 1o alcohol esterified to phosphate ● amino alcohol (= choline) Found in nerve membranes and brain 82 CERAMIDE 83 Phosphate Choline 84 GLYCOLIPID ● When a carbohydrate is attached to OH- via glycosidic bond ● Seb. induk = ceramide (sphingolipid) + CHO ● Cerebroside - CHO = glucose @ galactose ● glucocerebroside ● GANGLIOSIDE – ALSO contains oligosaccharide + sialic acid 85 86 DERIVED LIPIDS ● A heterogeneous group ● Derived from fatty acids ● steroids, prostaglandin, leukotriene, carotenoids, vitamin STEROID All organisms – similar basic structure – fused ring= perhydrocylopentanophenanthrene 87 88 89 STEROL i. Hydrocarbon chain (C18-C20) at C17 ii. Hydroxyl group (OH) at C3 ● Main example = CHOLESTEROL – structural component of membrane - 0 -40% lipid membrane. Rigid ● Precursor of bile, sex hormones, vit. D. ● Role in atherosclerosis 90 Hydrocarbon chain at C17 OH at C3 CHOLESTEROL 91 92 TERPENE ● Lipid derived from isoprene ● Term used for all compounds synthesized from the precursor isoprene cholesterol, bile acid, steroid, lipid soluble vitamins = terpene ● Oils from turpentine (pine tree extracts) ● formula C10H15 ● > 15 carbon atom also found - `multiples of 5 ● Also in other plants 93 TERPENE Terpene with 20 carbon atoms - vit. A - 40 carbon atoms - b- carotene EXAMPLES: 1. monoterpene - Limonene - `odor' lemon 2. Diterpene - Gibberrelic acid – plant hormone 3. Triterpene - Squalene – Cholesterol precursor 4. Tetraterpene - Lycopene - tomato pigments 94 TERPENE 95 TERPENE 96 TERPENE 97 BCM 3000 PRINCIPLES OF BIOCHEMISTRY (Semester 1 -2011/12) 98 LIPID BEHAVIOUR IN WATER ● Lipid – not soluble in water but can still be found in aqueous environment ● behavior in water important to understand the phenomena ● A lot of lipids are amphiphyllic = having hydrophobic part (hydrocarbon chain) polar (ionic) part 99 When lipid is dispersed in water, the hydrophobic part will segregate from the solvent through `self-aggregation' – form a. micelles – which are dispersed in water b. monolayers ( aggregate – boundary H2O: air 100 MICELLES MONOLAYER 101 The tendency for hydrocarbon chains to distance away from polar solvents gives rise to = HYDROPHOBIC EFFECT ● Most lipids will form micelles – spheres, ellipse, discs, cylinders ● Also can form vesicles – bilayer – hydrocarbon chains are opposite to each other `hollow sphere' 102 Micelle Vesicles Bilayer 103 BILAYER 104 Cholesterol does not form micelles ?? Not amphiphatic compounds Structure – flat fused ring - solid –difficult to form micelles Can form mixed micelle with amphiphatic lipids mixed micelles – with amphiphatic lipids 105 BILE ACID AND BILE SALTS Bile acids serve many functions. ● They aid in fat absorption ● Bile acids are produced from cholesterol in the liver. ● Cholesterol is converted to the carboxylic acids cholic and chenodeoxycholic acid, which are the primary bile acids in most species. ● The liver conjugates the acids to either glycine or taurine and subsequently secrets them into the bile. ● The gall bladder serves to store bile acids until contraction associated with feeding 106 Glycine Taurine 107 LIPOPROTEIN Particles that contain lipid and protein bonds = not (non-covalent) bonds Function – In blood plasma – to transport triacylglycerol and cholesterol STRUCTURE - form `micelle like particles' i. core – non-polar triacylglycerol ii. Surrounded by a layer of amphiphilic protein, phospholipid and cholesterol 108 Various categories – depending on the functions i. CHYLOMICRON – Carries exogenous triacylglycerols & cholesterol (from diet) from intestine to the tissues. ii. LDL, IDL & LDL – group of related particles which carry endogenous triacylglycerols & cholesterol (produced internally) from the liver to tissues NB: liver can synthesize triacylglycerol from excess carbohydrate 109 CHYLOMICRON 110 111 LDL, CHOLESTEROL & ATHEROSCLEROSIS Cholesterol – ● Important component of membrane – can be supplied from the outside or internally (if not enough) ● How obtained externally ? – ENDOCYTOSIS – Through reaction of specific receptors = LDL receptor? ● protein part of LDL tie up to R-LDL in the cell complex `pinched off' = endocytosis 112 ENDOCYTOSIS vs EXOCYTOSIS 113 114 Protein – recycled – used in the cell Oversupply ? – Synthesis of R-LDL inhibited low LDL cholesterol level in blood increases deposited in the artery heart disease; stroke HDL Function opposite of LDL Carries cholesterol from tissues - extract cholesterol from membrane – change to `cholesteryl esters - LCAT (Lecithin cholesterol transferase) bile acids 115