* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Supplement
Cytokinesis wikipedia , lookup
List of types of proteins wikipedia , lookup
Cell culture wikipedia , lookup
Tissue engineering wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell encapsulation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
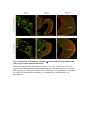
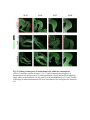
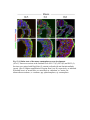
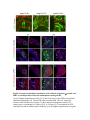
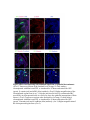
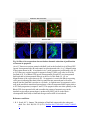
www.sciencemag.org/content/343/6176/1253/suppl/DC1 Supplementary Materials for Vertebrate Limb Bud Formation Is Initiated by Localized Epithelial-toMesenchymal Transition Jerome Gros and Clifford J. Tabin* *Corresponding author. E-mail: [email protected] Published 14 March 2014, Science 343, 1253 (2014) DOI: 10.1126/science.1248228 This PDF file includes: Materials and Methods Figs. S1 to S6 References Materials and Methods Immunostaining Embryos were embedded in 7.5% gelatin / 15% sucrose and sectioned using a Leica CM3000 cryostat. Sections were then incubated with primary antibodies in (PBS/BSA 0.2%, Triton 0,1% / SDS 0,02%) overnight, washed 2x10min in PBS and incubated for 30min in (PBS/BSA 0.2%, Triton 0,1% / SDS 0,02%) with secondary antibodies. Antibodies used were anti β−catenin (BD BioSciences), aPkc (Santa cruz), laminin (Sigma), Brdu (BD BioSciences) and Phalloidin (Invitrogen), Vimentin (Abcam), Ncadherin (BD BioSciences). Apoptosis was detected using the click-it Tunel detection kit from Invitrogen and Proliferation by incubation of embryos with a 10-2M BrdU solution. Cell counting was performed using ImageJ and the plugins “Colocalization” and “ITCN” written by Pierre Bourdoncle (Institut Jacques Monod, Paris) and Thomas Kuo (UCSB). Electroporation and expression constructs White leghorns chicken embryos were ordered form Charles River Laboratory. Electroporation was performed as previously described (13). Briefly, a plasmidic solution of DNA at 1µg. µl-1 was injected into the coelomic cavity of stage 13 embryos and 3 pulses of 5ms at 50V were applied using homemade electrodes. RhoA expression vector was kindly provided by Dr. Sheng (4); Fgf10 was cloned from a limb cDNA library and subcloned into a pCAG-ires-GFP construct. Mouse strains Fgf10 (11) and Tbx5 (9) mutant strains were used in this study, as previously published. Fig. S1. Expression of Ncadherin, Vimentin, β-catenin and aPKC throughout the early stages of chick limb bud formation. Transverse sections at the forelimb level of stage 13 (A, D), 15 (B, E) and 19 (C, F). Sections were immunostained with Ncadherin (green), Vimentin (red) in (A to C) and aPKC (green), β−catenin (red) in (D to F). nt: neural tube; no: notochord; en: endoderm; so: somite; im: intermediate mesoderm; ec: ectoderm; spp: splanchnopleure; sp: somatopleure. Fig. S2. Timing of emergence of mesenchymal cells within the somatopleure. (A to L) Transverse sections of stage 15, 16, 17 and 18 showing the emergence of mesenchymal cells as observed by F-actin organization (Green) and nuclear morphology (Dapi, white) at the forelimb (A to D), trunk (E to H) and hindlimb (I to L) level. Pictures of the stage at which mesenchymal cells were first observed for each region are framed in red. Fig. S3. Cellular state of the mouse somatopleure across development. (A to C) Transverse sections at the forelimb level of E8.75 (G), E9.0 (H) and E9.25 (I). Sections were stained with Dapi (blue), β-catenin (red) and with anti laminin antibody (green). (D to F) Higher magnification of region from (A to D), respectively, as indicated by dashed boxes. nt: neural tube; no: notochord; en: endoderm; so: somite; im: intermediate mesoderm; ec: ectoderm; spp: splanchnopleure; sp: somatopleure. Fig. S4: Changes in subcellular localization of N-cadherin, Vimentin, β-catenin and aPKC as electroporated cells of the somatopleure undergo EMT. (A to C) Higher magnification of Fig.1,G, H and I, focusing on the GFP electroporated epithelial somatopleure 3h, 12h and 24h after electroporation. (D to G) Transverse sections at the forelimb level of stage 13 chick embryo electroporated with a GFP reporter gene, re-incubated for 3 hours (D, E) or 24 hours (F, G) and stained for GFP, vimentin (red) and N-cadherin (blue) antibody. (H to K) Higher magnification of regions from (D to G), as indicated by dashed boxes. Note the basal localization of Vimentin and apical localization of N-cadherin in GFP epithelial somatopleure cells (yellow arrowheads and white arrows respectively) and relocalization of N-cadherin and increased Vimentin staining in GFP positive cells that have left the somatopleure epithelium (G to H, white arrowheads).(L to O) Transverse sections at the forelimb level of stage 13 chick embryo electroporated with a GFP reporter gene, re-incubated for 3 hours (L, M) or 24 hours (N, O) and stained for GFP (green), β-catenin (red) and aPKC (blue) antibody. (P to S) Higher magnification of region from (L to O), as indicated by dashed boxes. Note the specific enrichment of aPKC and β-catenin staining at the apical end of electroporated cells (white arrows) and relocalization of β-catenin and aPKC after cells have left the epithelium (R, S, yellow arrowheads). Fig. S5: Rhoa overexpressing cells maintain expression of epithelial determinants. (A to C) Transverse sections at the forelimb level of stage 15 chick embryo electroporated with RhoA and GFP, re-incubated for 24 hours and stained for GFP (green), β-catenin (red) and aPKC (blue) antibody. (D to F) Higher magnification of the electroporated region from (A to C). Note the increased levels of β-catenin and aPKC specifically in electroporated cells as cells fail to leave the epithelial somatopleure (White arrows). (G to I) Transverse sections at the forelimb level of stage 15 chick embryo electroporated with RhoA and GFP, re-incubated for 24 hours and stained for GFP (green), Vimentin (red) and N-cadherin (blue) antibody. (J to L) Higher magnification of the electroporated region from (G to I). Fig. S6: RhoA Overexpression does not induce dramatic reduction of proliferation or increase in apoptosis. (A to C) Transverse sections stained with BrdU (red) at the forelimb level of RhoA/GFP (green) electroporated side (B) and control un-electroporated side (A). (C) Magnification of the region shown in (B). Arrowheads in in C point at numerous proliferating cells. (D to G) Transverse sections assayed for apoptosis using TUNEL (red) at the level of the forelimb of (E, F) of RhoA/GFP (green) electroporated (D) and WT un-electroporated limb buds and un-electroporated embryos at the level of the flank (G). (F) is a Magnification of the region shown in (E). No apoptosis is seen in the RhoA-expressing cells (green) indicating that their failure to transform into mesenchymal cells and to migrate into the limb bud is not due to cell death. A slight increase in apopotosis can be seen in the non-RhoA transfected mesenchyme of the electroporated limb region relative to WT limb progenitors (compare E and F). This apoptosis does not relate spatially to the RhoA/GFP electroporated cells and resembles the pattern of apoptosis seen in the interlimb region where mesenchyme is produced by EMT, but (as in the RhoAelectroporated limb field) no limb bud emerges and an AER is not induced References and Notes 1. R. L. Searls, M. Y. Janners, The initiation of limb bud outgrowth in the embryonic chick. Dev. Biol. 24, 198–213 (1971). Medline doi:10.1016/0012-1606(71)900959 2. A. Mauger, [The role of somitic mesoderm in the development of dorsal plumage in chick embryos. I. Origin, regulative capacity and determination of the plumageforming mesoderm]. J. Embryol. Exp. Morphol. 28, 313–341 (1972). Medline 3. H. Ohuchi, T. Nakagawa, A. Yamamoto, A. Araga, T. Ohata, Y. Ishimaru, H. Yoshioka, T. Kuwana, T. Nohno, M. Yamasaki, N. Itoh, S. Noji, The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 124, 2235–2244 (1997). Medline 4. Y. Nakaya, E. W. Sukowati, Y. Wu, G. Sheng, RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat. Cell Biol. 10, 765–775 (2008). Medline doi:10.1038/ncb1739 5. J. P. Thiery, H. Acloque, R. Y. J. Huang, M. A. Nieto, Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009). Medline doi:10.1016/j.cell.2009.11.007 6. M. J. Cohn, J. C. Izpisúa-Belmonte, H. Abud, J. K. Heath, C. Tickle, Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739–746 (1995). Medline doi:10.1016/0092-8674(95)90352-6 7. H. Ohuchi, T. Nakagawa, M. Yamauchi, T. Ohata, H. Yoshioka, T. Kuwana, T. Mima, T. Mikawa, T. Nohno, S. Noji, An additional limb can be induced from the flank of the chick embryo by FGF4. Biochem. Biophys. Res. Commun. 209, 809–816 (1995). Medline doi:10.1006/bbrc.1995.1572 8. A. Vogel, C. Rodriguez, J. C. Izpisúa-Belmonte, Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development 122, 1737–1750 (1996). Medline 9. P. Agarwal, J. N. Wylie, J. Galceran, O. Arkhitko, C. Li, C. Deng, R. Grosschedl, B. G. Bruneau, Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 130, 623–633 (2003). Medline doi:10.1242/dev.00191 10. C. Rallis, B. G. Bruneau, J. Del Buono, C. E. Seidman, J. G. Seidman, S. Nissim, C. J. Tabin, M. P. Logan, Tbx5 is required for forelimb bud formation and continued outgrowth. Development 130, 2741–2751 (2003). Medline doi:10.1242/dev.00473 11. H. Min, D. M. Danilenko, S. A. Scully, B. Bolon, B. D. Ring, J. E. Tarpley, M. DeRose, W. S. Simonet, Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156–3161 (1998). Medline doi:10.1101/gad.12.20.3156 12. K. Sekine, H. Ohuchi, M. Fujiwara, M. Yamasaki, T. Yoshizawa, T. Sato, N. Yagishita, D. Matsui, Y. Koga, N. Itoh, S. Kato, Fgf10 is essential for limb and lung formation. Nat. Genet. 21, 138–141 (1999). Medline doi:10.1038/5096 13. J. Gros, J. K. Hu, C. Vinegoni, P. F. Feruglio, R. Weissleder, C. J. Tabin, WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr. Biol. 20, 1993–2002 (2010). Medline doi:10.1016/j.cub.2010.09.063