* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Engineering a Gene Silencing Viral Construct that Targets the
Community fingerprinting wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Gene regulatory network wikipedia , lookup
Bottromycin wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Exome sequencing wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
RNA silencing wikipedia , lookup
Genomic library wikipedia , lookup
RNA interference wikipedia , lookup
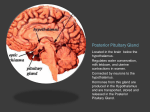
Close this window to return to IVIS www.ivis.org Proceedings of the 8th International Symposium on Canine and Feline Reproduction ISCFR June 22-25, 2016 Paris, France In a joint meeting with the XIX EVSSAR Congress Reprinted in IVIS with the permission of the ISCFR Organizers Reprinted in IVIS with the permission of the Organizers ISCFR VIII International Symposium on Canine and Feline Reproduction Close this window to return to IVIS PARIS, FRANCE JUNE 22-25, 2016 EVSSAR XIX Engineering a gene silencing viral construct that targets the cat hypothalamus to induce permanent sterility Gregory A. Dissena, Alejandro Lomniczia, Kei Adachib, Thomas Chatkupt c, Beverly L. Davidsond, Hiroyuki Nakaiab, and Sergio R. Ojedaa. a Division of Neuroscience, Oregon National Primate Research Center-Oregon Health & Science University, Beaverton, OR 97006; bDepartment of Molecular & Medical Genetics, cDepartment of Comparative Medicine, Oregon Health & Science University, Portland, OR 97239, USA;. dThe Children’s Hospital of Philadelphia, Philadelphia, PA, 19104, USA [email protected] We are using a combined approach to permanently sterilize cats. This approach employs two complementary methodologies: RNA interference (RNAi) to silence genes involved in the central control of reproduction; and a virus-based gene therapy system intended to deliver RNAi selectively to the hypothalamus (where these genes are expressed) via systemic administration of modified viruses. We selected the hypothalamus because it contains neurons expressing Kiss1, a gene essential for reproduction and fertility. We chose the non-pathogenic adeno-associated virus (AAV) as a vector whose tropism could be modified to target the hypothalamus. We first used a phage display library and a technique termed bio-panning to identify peptide sequences able to reach the hypothalamus. We next modified the tropism of the AAV vector by inserting the most promising peptide sequences into the viral capsid. Although we were able to re-target the AAV vector from the liver to the hypothalamus, the level of hypothalamic transduction was not enough to deliver RNAi at levels capable of reducing kisspeptin release and disrupt reproduction. To overcome this limitation we turned to a novel approach termed AAV Barcode-Seq1 that is allowing us to engineer and test hundreds of AAV mutants in a high throughput fashion using nextgeneration sequencing (NGS) technology. As a first step we performed a series of studies to determine the prevalence of anti AAV antibodies in cat serum derived from feral, client-owned and specific pathogen free (SPF) populations of cats (n = 94). The presence of antibodies targeting 11 common human serotypes of AAV was determined by ELISA. Overall, the cats with the highest prevalence of antibodies to AAV were client owned cats. Feral and SPF cats had comparably low levels of antibodies. Undetectable levels were found in immature SPF cats. Importantly, all cat populations had very low levels of antibodies targeting the serotype AAV9, suggesting that AAV9 would be a good platform to engineer mutants able to target the hypothalamus. The validity of this prediction was supported by the outcome of a study in which a DNA/RNA barcoded AAV library containing 58 AAV strains, including 11 common AAV serotypes (1-11) and a diversity of AAV mutants, was intravenously injected into two cats. Pharmacokinetic analysis of AAV clearance from blood revealed that AAV9 variants remain longer in the circulation than other AAV species. NGS analysis of a variety of peripheral tissues and brain regions allowed us to single out three AAV9 variants devoid of liver tropism. We are now using these variants as a platform for studies aimed at generating an AAV-based random octapeptide display library, which will be injected into naive cats to identify AAV mutants able to reach the hypothalamus selectively with minimal vector dissemination to other tissues. [1] Adachi K, Enoki T, Kawano Y, Veraz M, Nakai H. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nat Commun 2014; 5:3075 doi: 10.1038/ncomms4075 (2014) 51 Proceedings of the International Symposium on Canine and Feline Reproduction, 2016 - Paris, France