* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gene Therapy and Viral Vector
Transcriptional regulation wikipedia , lookup
Whole genome sequencing wikipedia , lookup
Community fingerprinting wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Molecular cloning wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Expression vector wikipedia , lookup
Non-coding DNA wikipedia , lookup
Molecular evolution wikipedia , lookup
Gene therapy wikipedia , lookup
Genome evolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
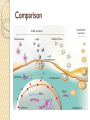
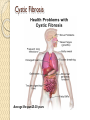
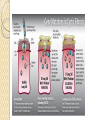
Gene Therapy and Viral Vector Lecture 4 Adeno-Associated Viral Vectors Adeno-associated viruses belong to the parvovirus family. Small viruses with a genome of single stranded DNA. These viruses can insert genetic material at a specific site on chromosome 19 with near 100% certainty. There are a few disadvantages to using AAV, including the small amount of DNA it can carry (low capacity) and the difficulty in producing it. This type of virus is being used, however, because it is non-pathogenic (most people carry this harmless virus). In contrast to adenoviruses, most people treated with AAV will not build an immune response to remove the virus and the cells that have been successfully treated with it. Comparison with Adeno Viruses Adeno-associated vectors (AAV) are like adenoviral vectors in their features but because of having some deficiency in their replication and pathogenicity, are safer than adenoviral vectors. In human, AAVs are not associated with any disease. Another special character of AAV is their ability to integrate into a specific site on chromosome 19 with no noticeable effects cause long-term expression in vivo. AAVs have been used in the treatment of some diseases, such as CF, hemophilia B, Leber congenital amaurosis, and AAT (Alpha-1 antitrypsine) deficiency. Structure of the Genome The AAV genome is built of single-stranded deoxyribonucleic acid (ssDNA), either positive- or negative-sensed, which is about 4.7 kilobase long. The genome comprises inverted terminal repeats (ITRs) at both ends of the DNA strand, and two open reading frames (ORFs): rep and cap (see figure 1). The former is composed of four overlapping genes encoding Rep proteins required for the AAV life cycle, and the latter contains overlapping nucleotide sequences of capsid proteins: VP1, VP2 and VP3, which interact together to form a capsid of an icosahedral symmetry. The Inverted Terminal Repeat (ITR) sequences comprise 145 bases each. They were named so because of their symmetry, which was shown to be required for efficient multiplication of the AAV genome. Another property of these sequences is their ability to form a hairpin, which contributes to so-called self-priming that allows primase-independent synthesis of the second DNA strand. The ITRs were also shown to be required for both integration of the AAV DNA into the host cell genome and rescue from it, as well as for efficient encapsidation of the AAV DNA combined with generation of a fully-assembled, deoxyribonuclease-resistant AAV particles. Recombinant Vector Genome The use of antisense oligodeoxynucleotides targeted to the reninangiotensin system [hormone system that regulates blood pressure and water (fluid) system] and adeno-associated virus vector delivery of antisense DNA offers a new approach to prolonged hypertension therapy with a single administration. Drawbacks The major disadvantages of these vectors are complicated process of vector production and the limited transgene capacity of the particles (up to 4.8 kb). Comparison Cystic Fibrosis Average life span:25-30 years Conventional Treatment The use of physiotherapy, antibiotics and pancreatic supplements. Many patients require treatment four times daily, including a considerable time spent on physiotherapy. This combination of treatment has helped increase life expectancy considerably to the current median of approximately 30 years of age. Despite small further improvements, more recently it has become apparent that there is a need for a more effective and convenient therapy. The identification of the gene responsible for CF (the cystic fibrosis transmembrane conductance regulator or CFTR protein) in 1989, was responsible for the advent of potential new treatments for CF. Gene therapy, the transfer of a normal copy of the CFTR gene into the lungs of CF patients, was proposed as an attractive new option. CFTR Pathway Cystic Fibrosis, a case study http://learn.genetics.utah.edu/content/gen etherapy/casestudy/ For further reading: http://www.intechopen.com/books/genetherapy-tools-and-potentialapplications/targeting-the-lung-challengesin-gene-therapy-for-cystic-fibrosis News of Gene therapy in CF http://www.bbc.com/news/scienceenvironment-32932922 http://www.nhs.uk/news/2015/07July/Page s/Gene-therapy-breakthrough-for-cysticfibrosis.aspx 3 July 2015 Lectures prepared from http://www.genetherapynet.com/viralvector/adeno-associated-viruses.html Further reading on AAV Efficient AAV Vector Production System: Towards Gene Therapy For Duchenne Muscular Dystrophy http://www.intechopen.com/books/genetherapy-tools-and-potentialapplications/efficient-aav-vectorproduction-system-towards-gene-therapyfor-duchenne-muscular-dystrophy