* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PERSISTENCE OF LEFT SUPERIOR VENA CAVA IN
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Myocardial infarction wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Coronary artery disease wikipedia , lookup
Electrocardiography wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
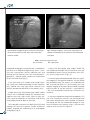
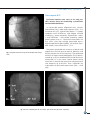
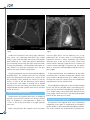
J Clin Med. 2010; 3(2):39-43 PERSISTENCE OF LEFT SUPERIOR VENA CAVA IN IMPLANTATION OF PERMANENT ELECTROCARDIOSTIMULATOR Sv. Iovev 1, Y. Dzhorgova1, B. Slavchev 1, M. Stoilova1, Ch. Panaiotov2 1 UNSHAT “St. Ekaterina” – Sofia 2 UMHAT “Aleksandrovska” - Sofia Author for contacts: Sv. Yovev, MU – Sofia, Clinic of Cardiology, Department of Electrocardiostimulation and Electrocardiophysiology, UNSHAT “St. Ekaterina”, 52A Pencho Slaveikov Blvd. – Sofia, Bulgaria; tel: +359 2 915 97 24, e-mail: [email protected] Case Reports: Persistent left superior vena cava (PLSVC) occurs in 0.3 percent of the total population, demonstrated in autopsy. This anatomical variant is usually discovered in patients during selective coronary angiography or procedures for temporary or permanent electrocardiostimulation. We present two clinical cases, in which PLSVC is used as a route for the introduction of leads for electrocardiostimulation. Case report №1 Persistent superior vena cava, as an additional route for introducing third stimulating lead, in patient with cardiac resynchronization therapy system A 58-year-old patient, diagnosed with “idiopathic dilated cardiomyopathy” (IDC), total heart failure (HF) and left bundle branch block (LBBB). The condition was discovered 7 years ago, when first symptoms of HF appeared. An invasive diagnostics was performed in 2009 – selective coronary angiography (SCAG), which excluded ischemic origin of the disease. The patient has been with acceleration of symptoms for one year, despite the optimal drug therapy with beta-blockers, cardiac glycosides, diuretics, antiarrythmics and anticoagulants. A course with Levosimendan administered twice daily was carried out, resulting in slightly improved global systolic function of left ventricle (LV) and temporary clinical effect. The patient entered the clinic with total heart failure, orthopnoea, hepatomegaly, low cardiac output condition. Catecholamine support was initiated with Dopamine to stabilize hemodynamic. Echocardiographic data (EchoCG) for diffuse hypokinesia - left ventricle ejection fraction (LVEF) - 10% with telediastolic volume of the left ventricle (TDV-LV) - 86 mm, telesystolic volume of the left ventricle (TSV-LV) 60 mm. Mitral insufficiency (MI) – 1st degree. By electrocardiography (ECG): sinus rhythm, LBB and duration of QRS complex 160 ms. The patient was evaluated as indicated for resynchronization therapy and a CRT system was implanted, model - “Frontier - I - DDDR”. Access - left subclavian vein (v.subclavia sin.) and vena cava superior. During the procedure, after the catheterization of the coronary sinus (CS), an introducer was inserted using electrophysiological catheter and an occlusive 39 Fig 1 Retrograde venography through the coronary sinus, demonstrates Fig 2 Anterograde venography – accessory vein, starting from the left the persistent left superior vena cava (PLSVC) and a postero-lateral subclavian vein and flowing into the coronary sinus with visualized leads branch are visualized of LV and RV PLSVC - Persistent Left Superior Vena Cava LV - Left Ventricle retrograde venography was performed. It revealed the presence of an additional large venous vessel – accessory vein, starting from the left subclavian vein and flowing into the coronary sinus, with well defined single branch - postero lateral, suitable for implantation of left ventricular lead (Fig. 1). A Left Ventricular (LV) unipolar lead, model “Quick site” (SJM), was introduced through the left subclavian vein, superior vena cava, right atrium, coronary sinus and the postero-lateral branch of the coronary sinus. A Right Ventricular (RV) bipolar lead, model “IsoFlex S 58cm” was introduced through left subclavian vein, superior vena cava, right atrium, apex of right ventricle. Optimal parameters of stimulation and sensing were programmed for both of the leads. The impossible insertion of the Right Atrial (RA) lead through the vena cava superior (smaller anatomical caliber), required the conducting of an anterograde venography (Fig.2). 40 RV – Right Ventricle A right atrial (RA) bipolar lead, model “Tendril 52” was introduced through the left subclavian vein and the additional venous way of the coronary sinus (PLSVC), into the right atrium (Fig 3, 4). In the early post procedural period, there was significant progress in the general condition. On the second post procedure day, the patient was mobilized and able to climb two floors. On the third day, the tissue Doppler imaging of the heart demonstrated improved pump function of the left ventricle – measured IFLV – 16%, with recovered intra-and inter- ventricular synchronicity. At the post procedural computed tomography (CT) scan with intravenous contrast, the three leads and their routes of placement were visualized. The lead for stimulating the RA was introduced through an anatomical variation of the venous system – persistent left superior vena cava (PLSVC) Figure 5. J Clin Med. 2010; 3(2):39-43 Case report №2 Persistent superior vena cava, as the only possible venous route for conducting a permanent electrocardiostimulation An 82-year-old patient diagnosed with: ischemic heart disease (IHD); stable angina pectoris (SAP) – II-III functional class (FC); hypertension disease – 3rd grade; moderate mitral insufficiency; high degree tricuspid insufficiency; severe pulmonary hypertension. chronic atrial fibrillation – tachy–brady syndrome; cardiac pauses greater than 3 s. Ventricular extrasystoles IVA class Lown. State after multiple presyncopal events. Right bundle branch block (RBBB). Left anterior hemiblok (LAHB). Heart failure NYHA - FC III. Fig 3 Retrograde introduction of right atrial stimulation lead through PLSVC The patient presented with shortness of breath and fatigue after minimal physical exercise, blackouts and fainting without loss of consciousness. The conducted 24 hours of Holter electrocardiography (ECG) demonstrated up to 35/min bradyarrhythmia and ventricular extrasystoles IV A class Lown, cardiac pauses lasting more than 3 seconds and ventricular tachyarrhythmia with frequency up to 160/min. Class I indications for implantation of permanent pace-maker (PM) – VVIR type. Fig 4 The three stimulating leads for left ventricle, right ventricle and right atrium, respectively 41 Fig 5 Under local anesthesia and using right subclavian vein access, we implanted permanent PM, model “Verify”, type VVIR and Right Ventricular (RV) bipolar lead “IsoFlex 58 cm. There were optimal parameters for stimulating and sensing the right ventricular lead. During the procedure, we discovered anomalous venous inflow in an accessory subclavian vein. The procedure itself went without complication. into the right atrium via the coronary sinus, in approximately 92% of cases, there is no hemodynamic expression and this is often combined with dilated coronary sinus. In the rest of the cases, it flows into the left atrium creating a right-left shunt. Anatomy of systemic venous drainage is important for the procedures in anesthesia, electrocardiostimulation and cardiac surgery. The post procedural contrast computed tomography revealed (fig.6): The superior vena cava was situated on the left, passing over the aortic arch, in front of the left pulmonary artery, behind the auricle of the left atrium, in front of the left pulmonary veins, behind the left atrium and under it flowing into a dilated coronary sinus near the inferior vena cava. Vena cordis magna flowed into the superior vena cava at the level of the left atrium. In the presented case, the introduction of the leads, stimulating the LV and RV through the superior vena cava, turned to be obstructive to its caliber, thus preventing the use of this venous route for introduction and placement of the third RA lead. Discussion of case report №1 The persistent left superior vena cava is a congenital anomaly (unsuccessful involution of the left cardinal vein). Its incidence in the population is about 0.3% – 0.5%. In 10% of the cases there is no right superior vena cava. When the persistent left superior vena cava flows 42 Fig 6 The retrograde introduction of the RA lead through PLSVC was the only possible route. Attempting to implant the left ventricular lead directly through PLSVC in LV would create a lot of difficulties, because of the sharp angle, which the PLSVC and the postero-lateral branch of the coronary sinus form. Discussion of case report №2 The persistent left superior vena cava is discovered incidentally, most often in implantation of temporary venous lead or in permanent electrocardiostimulation. The access used for the implantation (v.subclavia sin. J Clin Med. 2010; 3(2):39-43 or v.subclavia dex.) commonly determines the success of the procedure. In the presented case, we accidentally ran into this anatomical variation of a persisting left superior vena cava. When we used the route through the superior vena cava, there were some difficulties in introducing the lead in the right ventricle, due to the presence of unusual angle that forms between the coronary sinus and the right ventricle. The other problem in this case, was the insufficient length of the lead, because of the many additional curves in the described structures. The alternative access through left v.subclavia would provide better and easier placement of the lead in the right ventricle. Rarely these patients require implantation of an epimyocardial lead. 43