* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 7(Hill/Petrucci/McCreary/Perry Introduction to Atomic
Elementary particle wikipedia , lookup
History of quantum field theory wikipedia , lookup
Renormalization wikipedia , lookup
Ferromagnetism wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Particle in a box wikipedia , lookup
Chemical bond wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Matter wave wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic orbital wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Hydrogen atom wikipedia , lookup
Chapter 7(Hill/Petrucci/McCreary/Perry
Introduction to Atomic Structure
This chapter deals with the nature and properties of electrons and how electrons contribute to the
structure of atoms. We will begin by reviewing what we already know about electrons as
particles and then look at electrons as electromagnetic radiation. We will finish the chapter by
developing a more modern view of atoms, the quantum mechanical view.
“A body of knowledge … classical physics, was accumulated over several centuries up until the
early part of the twentieth century. This classical view included some ideas about atomic
structure” (Hill, p.260)
A Quick Review of Atomic Structure Up to 1900
- Crookes and “cathode rays”
- J.J. Thomson and the m/e ratio for the electron
- Millikan’s “oil drop” experiment and mass of an electron- – me = 9.109 x 10-28 g/electron and
charge on electron , e = -1.602 x 10-19 C (coulombs)
- Thomson’s atomic model: ”raisin pudding”
- Rutherford’s atomic model: the nuclear atom
] Read Sections 7.1 through 7.4, pp. 259-267 (Hill)
A white light source is comprised of all of the colors of the rainbow … the colors are related to
the energy of the radiation …Passing white light through a prism creates a continuous
spectrum.
Light And Electromagnetic Radiation
“WAVES” … Radiant energy includes sound, light and heat and is any form of energy that is
transferred by wave motion – but all waves have some common
properties … Ocean waves are both familiar and excellent examples of waves in general and of
wave properties in particular. Waves have two “components”: electric and magnetic !!
Visible light (with its range of rainbow- like colors ranging from red to violet) is only one part of
the electromagnetic “spectrum”. Electromagnetic radiation is radiant energy and
travels in waves (sine or cosine- like curves) …
All electromagnetic radiation is characterized by four variables: speed, wavelength, frequency,
and amplitude. In a vacuum, the speed is always 3.0 x 108 m/s. In other materials (like glass),
the speed of light is less than 3.0 x 108 m/s.
The radiant power (intensity) of the any radiation is directly proportional to the square of the
amplitude (A2 ) and is independent of the frequency or wavelength of the radiation.
The longer the wavelength (λ), the lower the frequency (n). Also, the energy (E) of the radiation
is directly proportional to the frequency, where, λ = c / ν and E = hν = hc / λ (Planck)
h = 6.626 x 10-34 J s (Planck’s constant)
Light energy travels in units called photons .
See Exercises 7.1A and 7.1B, p. 270, Hill …
Exercise 7.1A
Calculate the frequency if the wavelength is 1.07 nm
ν = c/λ c = 3.00 x 108 m s-1
ν = (3.00 x 108 m s-1 )/{(1.07 nm)(10-9 m/1 nm)} = 2.80 x 1011
ν = 2.80 x 1011 s -1 = 2.80 x 1011 Hz
Exercise 7.1B
Calculate the wavelength in nanometers if the frequency is 9.76 x 1013 Hz (s-1 ).
λ = c/ν c = 3.00 x 108 m s-1
λ = {(3.00 x 108 m s-1 )/(9.76 x 1013 s-1 )}·(1 nm/10-9 m)
λ = 3.07 x 103 nm
Thus, as wavelength, λ, increases (longer wavelength), both the frequency (ν) and the energy (E)
of the radiation decrease.
See “Electromagnetic Spectrum,” Figure 7.10, Hill, p. 269
See Exercise 7.3A, p. 274, Hill …
Calculate the energy, in joules per photon, for radiation with a frequency of 2.89 x 1010 Hz (s-1 ).
E = hν = hc / λ
h = 6.626 x 10-34 J s
-34
E = (6.626 x 10 J s)(2.89 x 1010 s-1 ) = 1.91 x 10-23 J/photon
See Exercise 7.4A, p. 274, Hill …
Calculate the energy, in kJ/mol, for visible light with a wavelength of 400 nm.
E = hν = hc / λ
h = 6.626 x 10-34 J s
First, convert 400 nm to meters (m): m = (400 nm)(10-9 m/nm) = 4.00 x 10-7 m
E = (6.626 x 10-34 J s)(3.00 x 108 m s-1 )/(4.00 x 10-7 m)
E = 4.97 x 10-19 J/photon
E = (4.97 x 10-19 J/photon)( 6.02 x 1023 photons/mol)
E = 2.99 x 105 J/mol = 2.99 x 102 kJ/mol = 299 kJ/mol
E = 299 kJ/mol of photons
Light and Quantum Theory
What is an electron? a particle with known mass and charge, me and e.
Particles with mass and charge obey classical laws of physics … Newton’s laws of motion .. k.e.
= ½ mv2 .
- Bunsen and line spectra (1860s) …emission spectrum for hydrogen gas
- Bright line spectra: atomic spectra … for elements! Emission spectra appear as bright line
spectra.
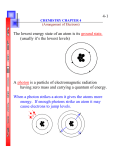
Classical Physics View of What Atoms Are (see Figure 7.16, Hill, p. 275)
The atom: classical physics .. e- spirals in to (+) nucleus .. with energy given off as light
NOT!
Bohr’s atom (1913): model for the hydrogen atom (see Figure 7.17, Hill, p. 276)
The atom: “quantized” energy levels for electrons … only certain energies “allowed”
Transitions between energy levels allowed when energy gained or lost by an electron
Light energy given off when electrons “drop” from higher to lower energy states.
Line Spectra and Energy Changes: The H-Atom
Label energy levels as integers, where n = 1, 2, 3, …
Energy of a given energy level: En = -B/n2 , where B = 2.179 x 10-18 J
Transition energy is difference between various energy levels :
∆E = Efinal – Einitial = - B (1/n2 final – 1/n2 iniital)
See Exercise 7.6A, p. 278, Hill
Calculate the energy change that occurs when an electron is raised from the n = 2 to the n = 4
energy level in a H-atom.
Solution. ∆E = - B (1/n2 final – 1/n2 iniital)
∆E = - (2.179 x 10-18 J)(1/42 – 1/22 )
∆E = - (2.179 x 10-18 J)(0.0625 – 0.250) = 4.1 x 10-19 J
∆E = hc/λ = 4.1 x 10-19 J = (6.626 x 10-34 J S)(3.0 x 108 m/s)/λ
or, λ = 4.85 x 10-7 m = 485 nm
Electron Transitions and Wavelength (see Example 7.8, p. 280, Hill)
Some of the electronic “transitions” for a hydrogen atom .. the Bohr model
The length of each of the arrows is proportional to the transition energy … regardless of whether
the transition is an absorption or an emission
Rank of transition energies: (a) > (b) > (d) > (c)
Rank of wavelengths: l ∝ 1/∆E …(a) < (b) < (d) < (c)
The Spectral Series for the Hydrogen Atom (see Figure 7.18, p.279, Hill)
Balmer: ni " n = 2 Lines in visible region of spectrum
Electronic Ground States and Excited States
For H-atom, the single electron usually resides in n = 1 (closest to nucleus) … atoms are in an
electronic ground state when their electrons are in the lowest possible energy levels.
When electrons in atoms are “kicked” up to higher energy levels, the atom is said to be in an
excited state. When electrons drop from higher, excited states back down to lower electronic
energy levels or to the ground state, they emit radiant energy as light.
The Concept of Particle-Wave Duality
Electrons have very small mass and travel at speeds close to speed of light … they exhibit
“nonclassical” behavior ….electrons act as if they are electromagnetic radiation instead of matter
… they have the properties of radiation, including wavelength …
De Broglie Wavelength (1923)
λ = (h/mv) for particle of mass = m and speed = v, where h = 6.626 x 10-34 J s
Ordinary objects have wavelengths too short to be observed … e.g. a 98 mph baseball …only 1.1
x 10-25 nm.
We use small particles like electrons and neutrons instead of visible light when we want to look
at very small objects, e.g. small structural details not revealed by an optical microscope …we
want l ≈ size of object.
Example. Calculate the wavelength of an electron traveling at 5.0 x 106 m s-1 . Recall me = 9.109
x 10-31 kg
λ = h/mv = (6.626 x 10-34 J s)/(9.109 x 10-31 kg)(5.0 x 106 m·s-1 )
λ = 1.5 x 10-10 m = 0.15 nm
From Bohr to Schrödinger … and Beyond…
The Bohr atom …a planetary model of the atom … electrons circulating around a nucleus in
circular orbits …employed a simple equation to predict the values of wavelengths for the bright
lines of the hydrogen spectrum … but wouldn’t work even for “simple” helium atoms – needed a
new approach.
Bohr model was built on classical Newtonian mechanics -- a new kind of mecha nics was
developed to treat small particles like electrons that traveled at high speeds approaching the
speed of light …
From Bohr to Schrödinger … Quantum Mechanics (QM) …
QM treated electrons like waves of energy instead of small particles – electrons exist in regions
of space (orbitals), not in orbits
Concept of “electron clouds”… The electron “cloud” represents the probability that the single
electron in the hydrogen atom is in any particular location at a given time.
Wave Mechanics and the Schrödinger Equation
Erwin Schrodinger (1926) … used quantum mechanics in place of classical Newtonian
mechanics to represent electrons as electromagnetic radiation (waves) … each electron in an
atom represented by a wave function (y) or wave equation … solution to the wave equation, a
complex differential equation, gives solutions, called quantum numbers, that represent unique
allowed energy states for each electron in an individual atom …
The quantum numbers for each electron in an atom are like an address that tells where they are
and what their energies may be. They specify the probability of finding an electron in a given
location and with a given energy.
Electron Clouds and Orbitals
Electrons are “located” in atoms in orbitals, regions of 3-D space, centered on the nucleus of a
given atom. Quantum numbers (there are four for each electron in an atom) from quantum
mechanics act as labels for both electron energies and electron locations in atoms…. these
include
1. principal quantum number (n) (shell)
2. secondary quantum number (l) (subshell)
3. magnetic quantum number (ml) (orbital)
4. spin quantum number (ms) (electron)
Allowed Values for Quantum Numbers : Limits on the values for n, l, ml and ms
n = 1, 2, 3, …. ∞
shell numbers
l = 0, 1, 2, 3, …. n – 1
sub-shell numbers
ml = -l, -l+1, …. 0, 1 ,2 ,3 … +l
orbital designation
ms = - ½ or ms = + ½
spin quantum number
Bohr Orbits or Shells
The picture of Bohr shells (orbits) is useful only because it portrays the physical significance of
“shells” - - shells represent the average distance of a certain “type” of electron from the atom’s
nucleus.
The Possible Values for n, l and ml (see Table 7.1, p. 284, Hill)
Quantum Numbers … “Addresses” of Electrons in Atoms
Number of “allowed” electrons by shell
Max. # electrons = 2 n2 for the n-shell
Example. n = 1, maximum electrons = 2 (12 ) = 2
Heisenberg Uncertainty Principle
The very act of “seeing” an electron involves the interaction of a photon with the electron,
changing its path and its position
“it is impossible to simultaneously determine both the exact position and the exact
momentum of an electron in an atom” ∆x·∆p ≥ h/2π (a constant)
This says we cannot build instruments that measure smaller and smaller … a lower limit exists!
Electron Probabilities and Orbital Shapes: 1s (see Figure 7.23, p. 287, Hill)
orbital = 3-D region of space where we can expect to find a given type of electron a certain
percentage of the time
Electron Probabilities and Orbital Shapes: 2s (see Figure 7.25, p. 287, Hill)
Pictorial Representation of p-Orbitals on a Nucleus (see Figure 7.26, p. 288, Hill)
“Pictures” of orbitals in a different type of sub-shell - - these represent the physical shape and the
orientation of allowed types of electron clouds that we call p-orbitals …l = 1, ml = -1, 0 and +1
(-l to +l)
Arbitrary assignment: px = -1, py = 0, pz = +1
Pictorial Representation of d-Orbitals on a Nucleus (see Figure 7.27, p. 288, Hill)
There are 5 d-orbitals in any d sub-shell
l = 2, ml = -2, -1, 0, +1, +2
Electron Spin and Spin Quantum Number
Electrons spin like a planet … clockwise and counterclockwise around an arbitrary north pole ..
two electrons may occupy any orbital, but must have opposed spins (+1/2 and -1/2) to minimize
electron repulsion due to (-) charges on each electron
Summary: Quantum Numbers and Electrons in Atoms
each electron in a given atom has a unique set of four quantum numbers
principal quantum number (n) … “shell” … represents average distance of electron in the shell
from the nucleus … distance increases with n- value (0, 1, 2, 3 …)
secondary quantum number (l) … “sub-shell” … represents the shape of the electron cloud … s, p-, d- or f- … l = 0 (s-orbital), l = 1 (p-orbital), l = 2 (d-orbital), l = 3 (f-orbital)
In a given shell, there can be only 1 s-orbital, 3 p-orbitals, 5 d-orbitals and 7 f-orbitals
magnetic quantum number (ml) … “orbital” … represents the orientation of the orbital electron
cloud in 3-D space along x, y, z coordinates … ml possibilities are dictated by the value of l (the
type of orbital) l =0, 0; l =1, -1,0,+1; l =2, -2,-1,0,+1,+2; l =3, -3,-2,-1,0,+1,+2,+3
spin quantum number (ms) … “spin” … represents the direction of electron spin … regardless of
type of orbital, only two possibilities (equal chance and energy), +1/2 or -1/2.
We’ll actually see how this works in Chapter 8, when we talk about electron configurations or
arrangements in atoms …..