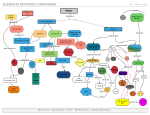
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Nuclear transmutation wikipedia , lookup
Transition state theory wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Biochemistry wikipedia , lookup
Atomic nucleus wikipedia , lookup
Water pollution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Acid–base reaction wikipedia , lookup
Chemical element wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electrochemistry wikipedia , lookup
Extended periodic table wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
History of chemistry wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Stoichiometry wikipedia , lookup
Water splitting wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrolysis of water wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Chemistry practice questions When copper and oxygen chemically unite, they form — F an ion G a gas H a compound J a mixture When copper and oxygen chemically unite, they form — H a compound Which of the following would most increase the rate at which magnesium nitrate dissolves in a liquid solvent? F Increasing the pressure on the liquid G Keeping the liquid still and undisturbed H Lowering the temperature of the liquid J Breaking the crystals into smaller pieces Which of the following would most increase the rate at which magnesium nitrate dissolves in a liquid solvent? J Breaking the crystals into smaller pieces Two students added a piece of zinc to a solution of hydrochloric acid in an open beaker. The mass of the products was found to be less than the total mass of the reactants. How should the students have conducted their experiment to reflect the law of conservation of mass? A the students should have used a less reactive metal in their experiment. B The students should have conducted the experiment in a closed system. C The students should have left the beaker on the scale during the entire reaction. D The law of conservation of mass does not apply to all chemical reactions, and the students’ results properly reflect that. Two students added a piece of zinc to a solution of hydrochloric acid in an open beaker. The mass of the products was found to be less than the total mass of the reactants. How should the students have conducted their experiment to reflect the law of conservation of mass? . B The students should have conducted the experiment in a closed system. Two students added a piece of zinc to a solution of hydrochloric acid in an open beaker. The mass of the products was found to be less than the total mass of the reactants. Which of the following statements best explains the loss of mass? A Zinc metal is more dense than the acid. B The chemical activity of the acid destroyed metal atoms. C A gas was released during the reaction. D Some of the zinc atoms failed to react. Two students added a piece of zinc to a solution of hydrochloric acid in an open beaker. The mass of the products was found to be less than the total mass of the reactants. Which of the following statements best explains the loss of mass? C A gas was released during the reaction. __Fe + __O2 → __Fe2O3 Iron reacts with oxygen in the air to form iron oxide. The unbalanced equation for this reaction is shown above. What are the coefficients when this equation is balanced? F 2,2,1 G 2,3,1 H 4,3,2 J 4,2,2 __Fe + __O2 → __Fe2O3 Iron reacts with oxygen in the air to form iron oxide. The unbalanced equation for this reaction is shown above. What are the coefficients when this equation is balanced? H 4,3,2 An inventor claims to have created a wind turbine that can convert 1 kilogram of water into 1 kilogram of oxygen and 1 kilogram of high-energy hydrogen fuel. 1 kg water → 1 kg oxygen + 1 kg hydrogen This claim is false because it — F violates the principle of constant composition G contradicts the law of conservation of matter H ignores the strength of the theory of strings J violates the rules of gravitational attraction An inventor claims to have created a wind turbine that can convert 1 kilogram of water into 1 kilogram of oxygen and 1 kilogram of high-energy hydrogen fuel. 1 kg water → 1 kg oxygen + 1 kg hydrogen This claim is false because it — G contradicts the law of conservation of matter Which of these steps in the digestive process is a physical change? F Water being absorbed in the large intestine G Enzymes breaking down proteins into amino acids H Saliva changing starches into sugars J Insulin breaking down simple sugars into smaller molecules Which of these steps in the digestive process is a physical change? F Water being absorbed in the large intestine Which characteristic of water molecules makes water a good solvent? F Mass G Polarity H Potential energy J Chemical energy Which characteristic of water molecules makes water a good solvent? G Polarity A student creates the above model of an atom. The overall charge of the atom represented in this model is B. Neutral Beryllium Be - - +++++ ++++++ 00000 - - - - Sodium (Na) - - +++++ ++++++ 00000 - - - - Substance Specific heat capacity (J/kg x oC) Aluminum 899 Copper 387 Gold 129 Silver 234 Water 4,184 Wood 176 Which of the following conclusions can be drawn from the information shown? A. B. C. D. Most types of matter heat up at the same rate. Most types of matter cool down at the same rate. It takes less energy to heat 1 g of silver then 1 g of gold. It takes more energy to heat 1 g of water then 1 g of copper. Substance Specific heat capacity (J/kg x oC) Aluminum 899 Copper 387 Gold 129 Silver 234 Water 4,184 Wood 176 Which of the following conclusions can be drawn from the information shown? A. It takes more energy to heat 1 g of water then 1 g of copper. Which of the following elements would be the best conductor? A. Au B. C C. Ne D. S Which of the following elements would be the best conductor? A. Au Which two elements are most similar? F. H and O G. Fe and Co H. Li and F J. Mg and Sr Which two elements are most similar? J. Mg and Sr The formula that represents sodium chloride, commonly known as salt, is – A. B. C. D. H 2O NaCl SaLt SoCh The formula that represents sodium chloride, commonly known as salt, is – B. NaCl This Bohr model represents the element – F. Carbon G. Fluorine H. Oxygen J. Neon This Bohr model represents the element – G. Fluorine What part of the oxygen atom contains neutral particles? A. B. C. D. Electrons Electron clouds Isotopes Nucleus What part of the oxygen atom contains neutral particles? D. Nucleus Which of the following must be the same for every atom of the element hydrogen? F. Atomic mass G. Number of electrons H. Number of neutrons J. Number of protons TAKS 3 8.8A, 8.8B TEKS Which of the following must be the same for every atom of the element hydrogen? J. Number of protons TAKS 3 8.8A, 8.8B TEKS You find an object that is shiny and solid. It conducts heat and electricity. How would the object be classified? A. B. C. D. noble gas non-metal metal halogen TAKS 3 8.9B TEKS You find an object that is shiny and solid. It conducts heat and electricity. How would the object be classified? C. Metal TAKS 3 8.9B TEKS Which of the following is not a property of an atom? F. Color G. Electric charge H. Mass J. Number of protons TAKS 3 8.8A, 8.8B TEKS Which of the following is not a property of an atom? F. Color Which of the following changes is not a physical change? A. B. C. D. Beating an egg Making a tossed salad Boiling water Frying an egg Which of the following changes is not a physical change? D. Frying an egg Which of the following would be a poor conductor of heat? a. F b. Ca c. Au d. Fe Which of the following would be a poor conductor of heat? a. F Which of the following is a naturally occurring chemical a. Salt b. Nylon c. Plastic d. Tylenol Which of the following is a naturally occurring chemical a. Salt According to the Periodic Table of the Elements, which element is most similar to magnesium (Mg)? A B C D calcium (Ca) iodine (I) sodium (Na) sulfur (S) According to the Periodic Table of the Elements, which element is most similar to magnesium (Mg)? A calcium (Ca) When Chemical X is added to a certain liquid, the chemical breaks into Substances Y and Z. It is not possible to break Substances Y and Z into simpler particles. Which statement is best supported by this evidence? A Chemical X is an element. B Chemical X is soluble in water. C Substances Y and Z are elements. D Substances Y and Z are compounds. When Chemical X is added to a certain liquid, the chemical breaks into Substances Y and Z. It is not possible to break Substances Y and Z into simpler particles. Which statement is best supported by this evidence? C Substances Y and Z are elements. The graph shows the relationship between mass and time for a radioactive element during radioactive decay. What is the half-life (time it takes for half of the element) to break down? A. 20 billion years B. 60 billion years C. 125 billion years D. 180 billion years The graph shows the relationship between mass and time for a radioactive element during radioactive decay. What is the half-life (time it takes for half of the element) to break down? A. 60 billion years