* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PowerPoint
Growth hormone therapy wikipedia , lookup
Metabolic syndrome wikipedia , lookup
Hypoglycemia wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Insulin resistance wikipedia , lookup
Gestational diabetes wikipedia , lookup
Diabetic ketoacidosis wikipedia , lookup
Complications of diabetes mellitus wikipedia , lookup
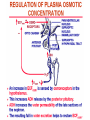
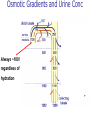
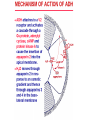
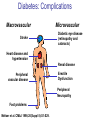
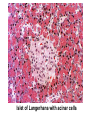
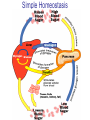
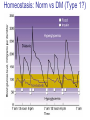
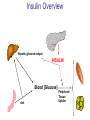
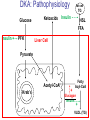
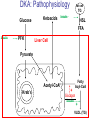
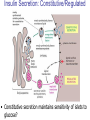
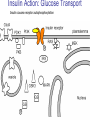
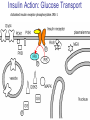
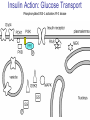
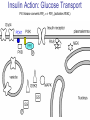
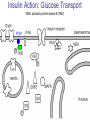
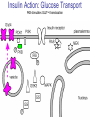
Pathophysiology of Brain & Body USSJJQ-20-3 Diabetes (part 1) “Diabetes” That which passes through… • Diabetes – Diabetes Insipidus (“…tasteless”) – Diabetes Mellitus (“…honey”) • Type 1 : Early Onset : IDDM • Type 2 : Late Onset : NIDDM • Atypical diabetes • Metabolic Syndrome Diabetes Insipidus (DI) Disorder arising from deficiency of anti-diuretic hormone (ADH) or its action copious amounts of dilute urine Rare, 0.01% of hospitalisations in US Central DI : failure of posterior pituitary gland to secrete adequate ADH Nephrogenic DI : renal tubules fail to respond to circulating ADH Inability of CT/CD to reabsorb water Lack of ADH-induced aquaporin 2 (‘water pores’) Osmotic gradient between medullary interstitium and filtrate still there, but cells of CD impermeable large volumes of dilute urine cellular and extracellular dehydration and hypernatremia Water (and solute) balance Net water efflux Net outward water movement Crenated Normal Hypertonic Swollen Isotonic Lysed Hypotonic Increasing ion concentration in ECF Bad Bad Less Water More Water Good Posterior Pituitary Composed of nerve fibres These neurosecretory cells synthesize Oxytocin & Vasopressin (ADH) cell bodies in hypothalamicparaventricular & supraoptic nuclei pass down nerve fibres & stored in/released from the posterior pituitary both nine-AA peptides ADH release stimulated by... Plasma osmolarity >280 mOsm/L Fall in plasma volume Emotional factors/stress Sleep Effect of ADH on nephron water permeability No ADH Water Permeability 100,000 + ADH 10,000 1,000 100 10 PCT tdL TAL DCT CCD IMCD Nephron Segment Osmotic Gradients and Urine Conc Always ~100! regardless of hydration Effects/Causes/Treatment Effects Causes (central) polyuria, polydipsia, nocturia/enuresis, hypernatraemic dehydration Idiopathic (30%) Tumours (30%) Infection, trauma, thyroiditis, leukaemia, genetic (AD, X-link) Treatment (central) Desmopressin (DDAVP) Causes (nephrogenic) Synthetic analogue Longer half-life More potent, more specific for renal effects familial: X-linked recessive Renal disease (chronic renal failure, polycystic) Treatment (nephrogenic) Adequate fluid intake, low sodium diet, diuretics Diabetes Mellitus (DM) Group of metabolic diseases characterised by hyperglycemia Results from defective insulin secretion and/or action Chronic hyperglycemia long-term damage/dysfunction/failure Main players… eyes, kidneys, nerves, heart, and blood vessels glucose glucose transporter insulin insulin receptor Increasing prevalence/burden ‘epidemic’ diasoce1.ppt 13 Diabetes: Complications Macrovascular Stroke Microvascular Diabetic eye disease (retinopathy and cataracts) Heart disease and hypertension Renal disease Peripheral vascular disease Erectile Dysfunction Peripheral Neuropathy Foot problems Meltzer et al. CMAJ 1998;20(Suppl 8):S1-S29. Diabetes: Burden Leading cause of blindness (12.5% of cases) Leading cause of End-Stage Renal Disease, ESRD (42% of cases) 50% of all non-traumatic amputations 2.5x increase risk of stroke 2-4x increase in cardiovascular mortality DM ‘responsible’ for 25% of cardiac surgery Mortality in DM: 70% due to Cardiovascular Disease Haffner et al, NEJM, 339(4):229-34, 1998. Diabetes: A Very Brief History 2nd Cent AD: “diarrhoea of the urine”... “the thirsty disease” Galen. “Diabetes is a wonderful affection, not very frequent among men, being a melting down of the flesh and limbs into urine” Aretaeus the Cappadocian 1850s autopsies suggest link with pancreas (patients with damaged pancreases almost always had DM) 1869: Paul Langerhans discovered existence of pancreatic acinar cells (pancreatic juice) and islets floating among the acini with an unknown function 1889: Minkowski & Von Mering depancreatized a dog polyuria as in diabetes. Ligating ducts did not DM (so not pancreatic juice) 1916: Paulescu normalized a diabetic dog with aqueous pancreatic extract 1955: Sanger’s group sequences bovine insulin Islet of Langerhans with acinar cells Insulin and Glucose Homeostasis Part of a larger picture… Organs… Glucose, Lipoprotein, Protein, Ketone metabolism Endocrine pancreas Adipose tissue, Liver, Gut, Skeletal muscle, Kidneys Hormones… Pancreatic hormones Insulin (β-cell) Glucagon (α-cell) Amylin (β -cell) Intestinal Hormones (Incretins) GLP-1 (L-cells) - Glucagon-like Peptide GIP (K-cells) - Glucose-dependent Insulin-releasing Peptide Insulin and Glucose Homeostasis Terms… Glycogenolysis Gluconeogenesis Ability of insulin to lower circulating glucose concentrations Insulin Resistance Production of ketone bodies ‘emergency’ fuel for heart/skeletal muscle Insulin Sensitivity Production of glucose from non-carbohydrates or proteins Ketogenesis Catabolism of glycogen Condition of low insulin sensitivity Honeymooning The ability of the failing β -cells to become hyper-productive and compensate for failing insulin response Simple Homeostasis Homeostasis: Norm vs DM (Type 1?) Glycosuria/Diuresis in DM Renal Filtered Load = [Plasma Gluc] x GFR Reabsorption is via facilitated transport Finite number of sites therefore Transport Maximum, TMax Hyperglycaemia increased Filtered Load When nears/exceeds TMax glucose remains in tubule Osmotic effects of glucose ‘trap’ water in tubule Get glucose in urine plus osmotic diuresis DM and Glycosuria Glucose Metabolism Major Metabolic Effects of Insulin Stimulates glucose uptake into muscle and adipose cells Inhibits hepatic glucose production Consequences of Insulin Deficiency Hyperglycemia osmotic diuresis and dehydration Insulin Overview Hepatic glucose output _ INSULIN Blood [Glucose] diet + Peripheral Tissue Uptake Lipoprotein Metabolism Major Metabolic Effects of Insulin Consequences of Insulin Deficiency Inhibits Elevated breakdown of triglycerides (lipolysis) in adipose tissue FFA levels Ketone Metabolism Major Metabolic Effects of Insulin Inhibits ketogenesis (Ketogenesis: is the process by which ketone bodies are produced as a result of fatty acid breakdown) Consequences of Insulin Deficiency Diabetic Ketoacidosis (DKA) DKA: Pathophysiology Glucose Ketoacids fat cell TG Insulin - HSL FFA Insulin + PFK Liver Cell Pyruvate Acetyl-CoA Kreb’s + Fatty Acyl-CoA Glucagon Insulin + VLDL (TG) DKA: Pathophysiology Glucose Ketoacids fat cell TG Insulin - HSL FFA Insulin PFK Liver Cell Pyruvate Acetyl-CoA Kreb’s + Fatty Acyl-CoA Glucagon Insulin + VLDL (TG) Protein Metabolism Major Metabolic Effects of Insulin Stimulates amino acid uptake and protein synthesis Inhibits protein degradation Regulates gene transcription Consequences of Insulin Deficiency Muscle wasting Overall catabolic rather than anabolic effects Insulin Secretion: Constitutive/Regulated • Constitutive secretion maintains sensitivity of islets to glucose? Insulin Secretion: Regulation by Glucose Insulin Action: Glucose Transport Insulin causes receptor autophosphorylation Insulin Action: Glucose Transport Activated insulin receptor phosphorylates IRS-1 Insulin Action: Glucose Transport Phosphorylated IRS-1 activates PI-3 kinase Insulin Action: Glucose Transport PI-3 kinase converts PIP2 => PIP3 (activates PDK1) Insulin Action: Glucose Transport PDK1 activates protein kinase B (PKB) Insulin Action: Glucose Transport PKB stimulates GLUT-4 translocation Bigger Picture: More than Insulin… Glucagon – glycogen breakdown, gluconeogenesis Adrenaline, noradrenaline – glycogen breakdown and gluconeogenesis in muscles, lactate glucose in liver Growth hormone (anabolic hormone), lipolysis, protesynthesis Glucocorticoids – gluconeogenesis, block of proteosynthesis Thyroid hormones and oestrogens Amylin – Co-secreted with insulin; anorectic (acts on brain ‘sated’ feeling) GLP-1 - “Incretin” hormone secreted by GI cells in response to a meal; +ve insulin secretion/sensitivity