* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Diapositiva 1
Molecular mimicry wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
DNA vaccination wikipedia , lookup
Immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
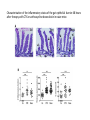
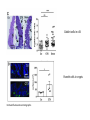
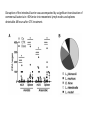
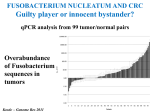
The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide by Sophie Viaud, Fabiana Saccheri, Grégoire Mignot, Takahiro Yamazaki, Romain Daillère, Dalil Hannani, David P. Enot, Christina Pfirschke, Camilla Engblom, Mikael J. Pittet, Andreas Schlitzer, Florent Ginhoux, Lionel Apetoh, Elisabeth Chachaty, PaulLouis Woerther, Gérard Eberl, Marion Bérard, Chantal Ecobichon, Dominique Clermont, Chantal Bizet, Valérie Gaboriau-Routhiau, Nadine Cerf-Bensussan, Paule Opolon, Nadia Yessaad, Eric Vivier, Bernhard Ryffel, Charles O. Elson, Joël Doré, Guido Kroemer, Patricia Lepage, Ivo Gomperts Boneca, François Ghiringhelli, and Laurence Zitvogel Science Volume 342(6161):971-976 November 22, 2013 Published by AAAS • Cyclophosphamide (CTX) is a clinically important cancer drug whose therapeutic efficacy is in part due to stimulate antitumor immune response. • In mice, they demonstrate that cyclophosphamide alters the composition of small intestine microbiota and induces the translocation of selected species of Gram+ bacteria into secondary lymphoid organs. • There, these bacteria stimulate the generation of “pathogenic” T helper 17 cells (pTh17) and memory Th1 immune responses. • Tumor-bearing mice either germ free or Gram+ bacteria depleted showed a reduction in pTh17 responses and their tumors were resistant to cyclophosphamide. • Adoptive transfer of pTh17 cells in these mice partially restored the antitumor efficacy of cyclophosphamide. The gut microbiota help shape the anticancer immune response. How do they demonstrate this? Characterization of the inflammatory status of the gut epithelial barrier 48 hours after therapy with CTX or anthracycline doxorubicin in naive mice. Goblet cells in villi Paneth cells in crypts Immunofluorescence micrographs Orally administered fluorescein isothiocyanate became detectable in the blood 18 hours after CTX treatment confirming an increase in intestinal permeability. Disruption of the intestinal barrier was accompanied by a significant translocation of commensal bacteria in >50% mice into mesenteric lymph nodes and spleens detectable 48 hours after CTX treatment. • QPCR was applied to determine the relative abundance, compared to all bacteria, of targeted groups of bacteria in the small intestine mucosa from CTXtreated/control, naive and tumor bearing mice. • In tumor bearers the total amount of bacteria 7 days after CTX treatment, as well as Clostridium leptum group, was not affected. • However there is a reduction in the abundance of lactobacilli and enterococci, revealing the capacity of CTX to provoke the selective translocation of some Gram positive species. • From previous works they know that CTX induces the polarization of splenic CD4 T cells toward a Th1 and Th17 pattern. • The gut microbiota is indispensable for driving the conversion of naive CD4 T cells into IL-17 producers is response to CTX. • Sterilization of the gut by broad spectrum antibiotics or treatment of mice with vancomycin, reduces the CTX induced Th17 conversion. IL-17 • In conventional SPF mice, the counts of lactobacilli and SFB measured in small intestine mucosa positively correlated with the Th1 and Th17 polarization of splenocytes. Not for Clostridium. • These results point to a specific association between particular microbial components present in the gut lumen and the polarity of TH responses induced by CTX treatment. CTX increases the frequency of “pathogenic” TH17 cells which share hallmarks of TH1 and TH17 cells within the spleen. This response is dependent on the gut microbiota. • They addressed whether Gram-positive bacterial species that translocated into secondary lymphoid organs in response to CTX could polarize naive CD4 T cells toward a TH1 and TH17 pattern. • Orally fed L. johnsonii and E. hirae facilitate the reconstitution of the pool of pTH17 cells in the spleen of ATB-treated SPF mice. • They postulate that the translocation of a specific set of Gram-positive commensal bacteria is necessary and sufficient to mediated the CTX-driven accumulation of pTH17 cells and TH1 bacteria-specific memory T cell responses. Effect of antibiotics on CTX-mediated tumor growth inhibition (P815 mastocytomas). • They monitored the effects of CTX against MCA205 sarcomas in different conditions. • They saw that in germ-free mice or treated with Vancomycin/Colistin, CTX works not so well in reducing the tumor size with respect to the control (SPF). • What they saw: specific CTX-induced alterations in gut microbiota-accumulation of pTH17 cells in the spleen-success of chemotherapy. •To establish a direct causal link between these phenomena they transferred TH17 or pTH17 populations into vancomycin-treated mice and evaluated their capacity to restablish the CTX-mediated tumor growth retardation. • Only pTH17, but not TH17 cells, could rescue the negative impact of Vancomycin on the CTX-mediated therapeutic effect. •The present study unveils the impact of gut microbiota on chemoterapy-elicited anticancer immune responses. • New risks associated with antibiotic medication during cancer treatments • Potential therapeutic utiliy of manipulating the gut microbiota Thank you!!