* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dana-Farber Cancer Institute | Spring 2002
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Cancer epigenetics wikipedia , lookup
BRCA mutation wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Frameshift mutation wikipedia , lookup
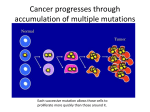
PROGRESS UPDATE The Prayers From Maria Foundation August 2015 EXECUTIVE SUMMARY As one of the leading experts in the study and treatment of pediatric brain tumors, Dana-Farber Cancer Institute is dedicated to advancing our understanding of these diseases and providing the finest patient care. New opportunities afforded by genomic analysis and other advancements are empowering our physician-scientists to learn more about the fundamental biology driving rare and challenging forms of disease. Accounting for approximately 10 percent of all pediatric nervous system tumors, diffuse intrinsic pontine gliomas (DIPGs) are highly aggressive and continue to present many treatment challenges. Under the direction of Mark Kieran, MD, PhD, Director of Pediatric Medical Neuro-Oncology, and Keith Ligon, MD, PhD, Director of Neuro-Oncologic Pathology, Dana-Farber investigators are leading the way in uncovering new information about the biological underpinnings of DIPG with the ultimate aim of improving outcomes for young patients with these tumors. This report highlights their ongoing efforts to understand the complex drivers of this disease in order to identify and evaluate the most promising novel targets and drugs. Your partnership plays a critical role in propelling this work and expediting the discovery of effective new therapies and treatment strategies. Mark Kieran, MD, PhD, Director of Pediatric Medical Neuro-Oncology LEADING THE WAY WITH DIPG BIOPSIES DIPGs develop in the pons, a highly sensitive area in the brain stem that, if injured, can cause serious side effects. In the 1980s, due to this challenge, surgeons ceased their attempts to biopsy the pons, limiting their ability to learn more about the development of DIPG and which drugs might be most effective against it. Since patients with DIPG almost always experience treatment resistance and disease recurrence, researchers need to learn more about the biology behind this disease to develop more impactful therapies. Keith Ligon, MD, PhD, Director of Neuro-Oncologic Pathology In 2002, acknowledging recent surgical and genomic analysis advances that would improve the safety of this procedure, Dr. Kieran began lobbying for physicians, scientists, and surgeons to reconsider biopsying patients with newly diagnosed DIPGs. Following more than 20 successful biopsies in 2007 by a medical team in France, Dr. Kieran had proof that DIPG biopsies could be done safely. In 2010, Dr. Kieran launched a large-scale clinical trial to biopsy 2 DIPGs at Dana-Farber and 24 other sites across the country, representing the first study of its kind in North America. Investigators conducted molecular analysis of these tumor samples and were sometimes able to make more informed treatment decisions based on their findings. Dr. Kieran and his team are now finalizing the results of the trial, which will clarify whether selecting DIPG treatments based on biopsies and analysis can improve survival. Since little was known about DIPGs prior to the trial, this tremendous new knowledge is expanding understanding of these rare tumors. In November 2014, Drs. Kieran and Ligon coordinated a work group session at the Society of Neuro-Oncology annual meeting, inviting participants to present innovative research strategies for DIPG based on the results of their upfront biopsies studies. Drs. Kieran and Ligon are now drawing on the findings and knowledge gained from the first study as they plan a new, more informed clinical trial. UNDERSTANDING DIPG THROUGH GENOMIC STUDIES Dana-Farber investigators are leveraging this expanded access to DIPG samples to better understand the drivers of this disease. Drs. Kieran and Ligon are collaborating with researchers in the United States and internationally to conduct genomic sequencing on these collections of DIPG tumors. In the April 2014 Nature Genetics, they published findings detailing their discovery of novel mutations linked to the development of DIPG, including in the genes ACVR1, FGFR1, PIK3CA, and PDGFRA. The mutation to ACVR1 was particularly significant since this mutation had never before been identified in cancer. The investigators continue to advance this work by evaluating these new targets and learning more about their complex role in driving DIPG. Dana-Farber investigators are also using DIPG samples to develop sophisticated laboratory models that closely recapitulate actual patient tumors, allowing investigators to study DIPG in a realistic model environment. This work can help researchers to pinpoint more effective drugs to test in patients, and could empower investigators to understand the diversity of patient responses to various drugs. Using these models, investigators recently discovered that even though almost all DIPGs have a histone mutation (see sidebar), these mutations do not appear to cause DIPG on their A histone is a type of protein that provides structural support to chromosomes. For DNA molecules to fit into the cell nucleus, they wrap around histones. Some histones also play a role in regulating gene expression. Image credit: Darryl Leja, NHGRI www.genome.gov 3 own. Drs. Kieran and Ligon aim to learn which combinations of mutations lead to DIPG and which arise during treatment, enabling them to elucidate the genomic landscape of this complex disease. These discoveries are already driving the development of novel therapeutic strategies for DIPG. Since existing drugs can target PI3K and PDGFRA mutations, the team is establishing clinical trials to explore the possibility of repurposing compounds to inhibit these alterations in DIPG. In collaboration with other Boston-area scientists, the team also created a novel diagnostic test that can identify patients with specific histone mutations that have been linked to poor outcomes. In the November 2014 Acta Neuropathologica, Drs. Kieran, Ligon, and their collaborators published their findings about the efficacy of this method, which is now used for clinical testing to screen for patients these mutations. EXPLORING DIPG AT A SINGLE CELL LEVEL Through these studies, Dana-Farber researchers are learning that DIPGs are highly heterogeneous and complex, which can make them challenging to treat (see sidebar). One drug might only knock out the tumor cells driven by one mutation, leaving other cancer cells to survive and continue growing. To overcome this challenge, Dana-Farber investigators are exploring combinations of targeted therapies. However, traditional “bulk” sequencing methods—which identify all of the cancer-promoting mutations in a tumor sample—can only give researchers a snapshot of the diversity in a tumor. While often effective in less complex cancers, bulk sequencing cannot indicate whether mutations are common to all tumor cells. To uncover the best ways to treat complex DIPGs, investigators need to identify not only the collective mutations, but the specific mutations within individual tumor cells. In the August 2014 Cancer Discovery, Dr. Ligon and his collaborators published a study aimed at addressing this problem in glioblastoma (GBM), another aggressive and complex brain tumor related to DIPG. Working with the laboratory of Matthew Meyerson, MD, PhD, Director of Dana-Farber’s Center for Cancer Genome Discovery, Dr. Ligon and his team developed a new singlecell sequencing method to identify specific DNA mutations within individual GBM tumor cells. This pioneering strategy—which provides a new, higher Mutation A Mutation B Mutation C Mutation D Mutation D Mutation A Mutation C Mutation A Mutation B Heterogeneity— genetic and molecular variability— is a common feature of malignant tumors. This means that the mutations that drive growth in one DIPG tumor cell might be completely different from the mutations driving cancer in a nearby cell—even within the same tumor. Top: Bulk analysis may reveal the presence of multiple mutations within a sample, but it doesn’t reveal how those mutations are distributed in sub-populations of cancer cells within the tumor. Bottom: Single-cell analyses allow scientists to identify tumor subpopulations bearing distinct combinations of mutations. 4 level of resolution for scientists to examine the mutations driving cancer— specifically revealed that a single GBM tumor is comprised of diverse populations of cancer cells bearing different cancer-promoting mutations. This information is already helping investigators to identify the mutations that contribute to GBM and fine-tune treatment strategies accordingly. Dr. Ligon and his colleagues are now exploring ways to apply single cell sequencing technology to learn more about the complex drivers of DIPGs. This strategy could allow investigators to understand the interplay between DIPG cancer cells and how they work together to drive tumor growth. This new knowledge will empower physicians to assemble the most promising combinations of therapies and deliver maximally effective treatments. A SINCERE THANK YOU Your meaningful commitment has helped Dana-Farber investigators to establish pioneering projects that advance our understanding of DIPGs. Their discoveries strengthen the foundation for uncovering new targets and improving treatment plans, keeping Dana-Farber at the forefront of pediatric cancer research and care. On behalf of our patients, thank you for your generous support in these critical efforts. Report written by Brittany Flaherty. Due to the sensitive location of DIPGs, researchers cannot collect large or numerous tissue samples, making it difficult to conduct broad analysis. To reduce the amount of tissue needed, the team recently developed a new technique to analyze various components in a single tumor sample rather than requiring multiple samples. “We are the ones developing this technology for DIPG. There is no place where the micro scale is as important as in DIPG samples, which are a millimeter in size.” - Dr. Mark Kieran The image above demonstrates the relative size of one millimeter. Image credit: Teresa Winslow FOR MORE INFORMATION Amy Trapasso Director, Corporate and Foundation Relations (617)-632-6601 [email protected] © 2015 Dana-Farber Cancer Institute. All Rights Reserved. No part of this report may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by an information storage or retrieval system, without permission in writing from Dana-Farber Cancer Institute. For additional information, please contact Jane Anderson at [email protected] or 617-632-5283. 10% of all designated gifts will support our Faculty Research Fund to advance Dana-Farber’s research mission. 5