* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Background radiation wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fission wikipedia , lookup
Isotopic labeling wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear drip line wikipedia , lookup
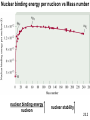
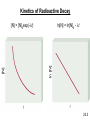
PowerPoint Lecture Presentation by J. David Robertson University of Missouri Nuclear Chemistry Chapter 23 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. REACTIONS CHEMICAL NUCLEAR RADIOACTIVE DECAY NUCLEAR TRANSMUTATION Unstable nuclei emits particles and/or electromagnetic radiation spontaneously. Bombardment of nuclei by neutrons, protons or other nuclei; occurrence are either naturally or artificially. 23.1 Atomic number (Z) = number of protons in nucleus Mass number (A) = number of protons + number of neutrons = atomic number (Z) + number of neutrons Mass Number Atomic Number proton neutron 1p 1H 1n or 0 1 1 A ZX Element Symbol electron 0b 0e or -1 -1 positron 0b 0e or +1 +1 a particle 4He 4a or 2 2 A 1 1 0 0 4 Z 1 0 -1 +1 2 23.1 Radioactive Decay A stable nucleus remains intact, but the majority of nuclei are unstable. An unstable nucleus exhibits radioactivity – it spontaneously disintegrates or decays, by emitting radiation. Each type of unstable nucleus has a characteristic rate of radioactive decay – might range from a fraction of a second to several billion years. “when a nuclide of one element decays, it changes into a nuclide of a different element” 23.2 When a nuclide decays, it forms a nuclide of lower energy, and the excess energy is carried off by the emitted radiation. The decaying nuclide is called ‘the parent’ and the product nuclide is called ‘the daughter’. Type of Radioactive Decay Alpha decay: loss of an α particle 212 4 208 A decreases by 4 84 2 82 Z decreases by 2 Po He Pb Beta decay: ejection of a β particle A does not change 40 40 0 19 K 20 Ca 1 b Z increases by 1 Positron decay: emission of a positron Positron is the ‘antiparticle’ of the electron. Positron decay has the opposite effect of β decay. C B b 11 6 11 5 0 1 A does not change Z decreases by 1 23.2 Electron capture: Occurs when the nucleus of an atom draws in an electron from the lowest energy level. The symbol is used to distinguished it from a β particle. A does not change 37 0 37 18 Ar 1 e17 Cl Z decreases by 1 Gamma Emission Loss of a -ray (high-energy radiation) that almost always accompanies the loss of a nuclear particle. Balancing Nuclear Equations 1. Conserve mass number (A). The sum of protons plus neutrons in the products must equal the sum of protons plus neutrons in the reactants. 235 92 U + 10n 138 55 Cs + 96 37 Rb + 2 10n 235 + 1 = 138 + 96 + (2x1) 2. Conserve atomic number (Z) or nuclear charge. The sum of nuclear charges in the products must equal the sum of nuclear charges in the reactants. 235 92 U + 10n 138 55 Cs + 96 37 Rb + 2 10n 92 + 0 = 55 + 37 + (2x0) 23.1 QUESTION #1: a) What radioactive isotope is produced in the following bombardment of boron? Answer: Note: Identify the element using the atomic number (proton number). Refer to the Periodic Table. b) Write the nuclear equation for the beta emitter Co–60 . Answer: Note: ‘Beta emitter’ means the reaction emits/produces beta particles. c) Complete the following nuclear reactions: a) b) 40Ar(a, p)? 59Co(n, a)? Answer: Mn Question #2: 212Po decays by alpha emission. Write the balanced nuclear equation for the decay of 212Po. 4 4 Alpha particle: 2 He or 2 a 212 84 Po He 4 2 208 82 X Checking the Periodic Table for the element with atomic number (proton number) of 82 gives the answer as Pb. Therefore: 212 84 Po He 4 2 208 82 Pb 23.1 Question #3: In the following reactions, identify X: 7 (a) 10 5 B ( X , a ) 3 Li (b) 238 92 U (15n, X ) 253 99 Es Answer: 5 B X a Li (a) 10 4 2 7 3 U 15 n X Es (b) 238 92 1 0 253 99 X n 1 0 X 7 b 0 1 Nuclear Stability & Mode of Decay Neutron-proton ratio (n/p ratio): Any element with more than one proton (i.e., anything but hydrogen) will have repulsions between the protons in the nucleus. A strong nuclear force helps keep the nucleus from flying apart. *Nuclear force = the force between nucleons i.e.: protons & neutrons Neutrons play a key role stabilizing the nucleus. Therefore, the ratio of neutrons to protons (n/p) is an important factor in determining the stability of nuclides. For smaller nuclei (Z 20) stable nuclei have a neutron-to-proton ratio close to 1:1. As nuclei get larger, it takes a greater number of neutrons to stabilize the nucleus. The shaded region in the figure shows what nuclides would be stable, the so-called belt of stability. Nuclei above this belt have too many neutrons. They tend to decay by emitting beta particles. Nuclei below the belt have too many protons. They tend to become more stable by positron emission or electron capture. 23.2 Nuclear Stability Certain numbers of neutrons and protons are extra stable: • Nuclei with n or p = 2, 8, 20, 50, 82 and 126 (magic numbers); • Extra stable numbers of electrons in noble gases (e = 2, 10, 18, 36, 54 and 86). Nuclei with even numbers of both protons and neutrons are more stable than those with odd numbers of neutron and protons. 23.2 All isotopes of the elements with atomic numbers higher than 83 are radioactive. All isotopes of Tc (Technetium) and Pm (Promethium) are radioactive. 23.2 Question #4: Which of the following nuclides would you predict to be stable and which is radioactive? Explain. 18 10 Ne 32 16 S 236 90 Th 123 56 Ba Answer: 18 10 Ne Radioactive. The n/p = 0.8. Despite having even number of proton and neutron, the minimum ratio for stability is 1.0. This nuclide has too few neutron to be stable. 32 16 S Stable. The n/p = 1.0 and it has even number of proton and neutron. 236 90 Radioactive. Every nuclide with Z > 83 is radioactive. 123 56 Radioactive. The n/p = 1.20, therefore this nuclide is not stable. Th Ba Nuclear Binding Energy Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up the nucleus. This mass difference is called the mass defect or mass deficiency, next, Einstein's formula: E = mc2 can be used to compute the binding energy of any nucleus. Nuclear binding energy per nucleon vs Mass number nuclear binding energy nucleon nuclear stability 23.2 Calculation Steps of Binding Energy per Nucleon: Step 1: Calculate mass defect. Mass defect is the difference between the mass of an atom and the sum of the masses of its protons and neutrons. Unit is in amu. Step 2: Convert amu to kg. Step 3: Calculate nuclear binding energy, BE. Use the formula: E = mc2 . Step 4: Divide binding energy by number of nucleons to get nuclear binding energy per nucleon. Question #5: Iron-56 is an extremely stable nuclide. Calculate the binding energy (BE) per nucleon for iron-56. Mass of 56Fe atom = 55.934939 a.m.u Mass of proton = 1.007825 a.m.u Mass of neutron = 1.008665 a.m.u Answer: 56 25 Fe 25 p 31 n 1 1 1 0 Step 1: Calculate mass defect. Mass defect, m = mass product – mass reactant = (mass p + mass n) – mass 56Fe mass p+n = (25 x 1.007825 amu) + (31 x 1.008665 amu) m = 56.46424 amu – 55.934939 amu = 0.529301 amu 23.2 Step 2: Convert amu to kg. m = 0.529301 amu x m = 8.789 x 10-28 kg 27 1.6605 10 kg 1.amu Step 3: Calculate nuclear binding energy, BE. Binding energy, BE: E = mc2 = 8.789 x 10-28 kg x (3.00 x 108 m/s)2 E = 7.91 x 10-11 J Step 4: Divide BE by number of nucleons. Binding energy per nucleon: E 7.911011 J 12 1.4110 J / nucleon # nucleon 56.nucleon Question #6: What is the nuclear binding energy per nucleon for 25Mg? Mass of 25Mg = 24.985839 a.m.u Mass of proton = 1.007825 a.m.u Mass of neutron = 1.008665 a.m.u Answer: 25 12 Mg 12 p 13 n 1 1 1 0 Mass defect, m = mass product – mass reactant = (mass p + mass n) – mass 25Mg mass p+n = (12 x 1.007825 amu) + (13 x 1.008665 amu) m = 25.206545 amu – 24.985839 amu = 0.220706 amu 23.2 m = 0.220706 amu x m = 3.665 x 10-28 kg 27 1.6605 10 kg 1.amu Binding energy, BE: E = mc2 = 3.665 x 10-28 kg x (3.00 x 108 m/s)2 E = 3.30 x 10-11 J Binding energy per nucleon: E 3.30 10 11 J 1.32 10 12 J / nucleon # nucleon 25.nucleon Kinetics of Radioactive Decay For each duration (half-life), one half of the substance decomposes. For example: Ra-234 has a half-life of 3.6 days. If you start with 50 grams of Ra-234: After 3.6 days 25 grams After 7.2 days 12.5 grams After 10.8 days 6.25 grams Half-Life The time taken for ½ of a sample to decompose. The rate of a nuclear transformation depends only on the “reactant” concentration. All radioactive decays obey first order kinetics. Kinetics of Radioactive Decay N daughter N rate = t rate = lN N = lN t N = N0exp(-lt) ln N = ln N0 - lt N = the number of atoms at time t N0 = the number of atoms at time t = 0 l is the decay constant ln2 l = t½ 23.3 Kinetics of Radioactive Decay ln[N] = ln[N]0 - lt ln [N] [N] [N] = [N]0exp(-lt) 23.3 Question #7: The half-life of radium-226 is 1.60 103 years. How many hours will it take for a 2.50 g sample to decay until 0.185 g of the isotope remains? Answer: ln 2 ln 2 4 1 l 4.33 10 years 3 t1 1.60 10 years 2 ln Nt = ln N0 - lt ln 0.185 = ln 2.50 – (4.33 x 10-4 yrs-1)t t = 6013 years or 5.27 107 hours Question #8: If 12% of a certain radioisotope decays in 5.2 years, what is the half life of this isotope? Answer: ln Nt = ln N0 - lt If N0 = 1.00 then Nt = 1.00 – 0.12 = 0.88 ln 0.88 = ln 1.00 – l(5.2) l = 0.0246 years–1 ln 2 ln 2 t1 28.2 years 1 2 l 0.0246 years Natural Radioactivity The figure shows the Decay Series. 238U Radioactive decay series is a sequence of nuclear reactions that ultimately results in the formation of a stable isotopes. The beginning radioactive isotope is called the parent and the product is called the daughter. Natural Radioactivity : Radiocarbon Dating for Determining the Age of Artifacts Natural Radioactivity : Radiocarbon Dating for Determining the Age of Artifacts Radioactive C-14 is formed in the upper atmosphere by nuclear reactions initiated by neutrons in cosmic radiation 14N + 1 n ---> 14C + 1H o The C-14 is oxidized to CO2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t1/2 = 5730 years. Activity of a sample can be used to date the sample. Natural Radioactivity : Dating based on Radioactive Decay Dating using uranium-238 isotopes: Some of the intermediate products in the uranium decay series have very long half-lives, therefore they are suitable for estimating the age of rocks in the earth and of extraterrestrial objects. Dating using potassium-40 isotopes: The radioactive potassium-40 isotope decays by several modes but the important one is of that electron capture. This particular mode can be use to gauge the age of a specimen in geochemistry.