* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 35_Organ-specific autoimmune diseases
Monoclonal antibody wikipedia , lookup
Innate immune system wikipedia , lookup
Rheumatic fever wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Atherosclerosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Germ theory of disease wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Globalization and disease wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Molecular mimicry wikipedia , lookup
Myasthenia gravis wikipedia , lookup
Autoimmunity wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
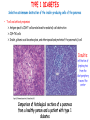
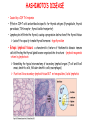
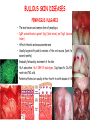
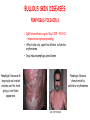
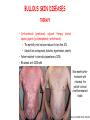
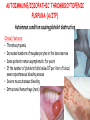
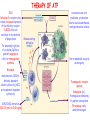
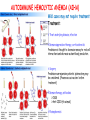
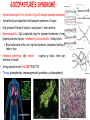
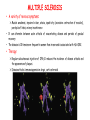
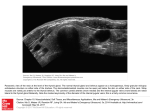
ORGAN-SPECIFIC AUTOIMMUNE DISEASES In organ-specific diseases, autoantigens from one or a few organs are targeted, and disease is limited to those organs AUTOIMMUNE ENDOCRINE DISEASES TYPE 1 DIABETES Selective autoimmune destruction of the insulin-producing cells of the pancreas • T-cell and antibody responses Antigen-specific CD8+T-cells are believed to mediate β-cell destruction CD4+Th1 cells Insulin, glutamic acid decarboxylase, and other specialized proteins of the pancreatic β-cell Insulitis: infiltration of lymphocytes from the islet periphery toward the center Comparison of histological sections of a pancreas from a healthy person and a patient with type 1 diabetes TYPE 1 DIABETES Selective autoimmune destruction of the insulin-producing cells of the pancreas • The β-cells comprise about two-thirds of the islet cells; as they die, the architecture of the islet degenerates. 108 β-cells - disease symptoms do not manifest until years • Disease symptoms usually manifest themselves in childhood Polyuria (excessive urination), polydipsia (increased thirst), xerostomia (dry mouth), polyphagia (increased hunger), fatigue, weight loss Diabetic ketoacidosis: xeroderma (dry skin), rapid deep breathing, drowsiness, abdominal pain, vomiting • Treatment: daily injection with synthetic human insulin; (coma, death) • Type 1 diabetes principally affects populations of European origin, 1 in 300. DQ2, DQ8 allotypes confer susceptibility to type 1 diabetes. DQ6 allotype confers strong resistance to type 1 diabetes. GRAVES’ DISEASE Production of thyroid hormones (thyroxine (T4), triiodothyronine (T3)) is regulated by thyroidstimulating hormone (TSH). AGONIST autoantibodies specific for the TSH receptor CHRONIC OVERPRODUCTION OF THYROID HORMONES The formation of autoantibodies driven by a CD4+Th2 response Graves’ disease is associated with HLA-DR3 (DR7 seems to be protective) GRAVES’ DISEASE Hyperthyroid condition: • Heat intolerance, rapid heart rate, nervousness, irritability, warm moist skin, weight loss, and enlargement of the thyroid • Graves’ ophthalmopathy Autoantibodies made against a thyroid protein cross-react with an eye-muscle protein. Fibroblast – glycosaminoglycan release – edema • Dermopathy – TSH receptor expressing skin fibroblasts Therapy: • Short-term treatment: inhibition of the production of thyroid hormones (inhibitor of thyroperoxidase). • Long-term treatment: radioactive iodine or surgery - destroy or remove the gland - need for lifelong use of replacement of thyroid hormones Temporary symptoms of antibody-mediated autoimmune diseases can be passed from affected mothers to their newborn babies. TSHR, thyroid-stimulating hormone receptor HASHIMOTO’S DISEASE • Caused by a CD4 Th1 response • Effector CD4+T-cells and antibodies specific for thyroid antigens (thyroglobulin, thyroid peroxidase, TSH receptor, thyroid iodide transporter) • Lymphocytes infiltrate the thyroid, causing a progressive destruction of the thyroid tissue Loss of the capacity to make thyroid hormones - hypothyroidism • Ectopic lymphoid tissues: a characteristic feature of Hashimoto’s disease: immune cells infiltrating the thyroid gland become organized into structures - lymphoid neogenesis - driven by lymphotoxin Resembling the typical microanatomy of secondary lymphoid organs (T-cell and B-cell areas, dendritic cells, follicular dendritic cells, macrophages) Functions like a secondary lymphoid tissue BUT not encapsulated, lacks lymphatics HASHIMOTO’S DISEASE Most common symptoms: • Fatigue, weight gain, feeling cold, joint and muscle pain, depression, panic disorder, slowed heart rate, irregular periods, problems getting pregnant and maintaining pregnancy • HLA DR4 association Treatment: • Replacement therapy with synthetic thyroid hormones taken orally on a daily basis. AUTOIMMUNE ADRENOCORTICAL FAILURE, OR ADDISON'S DISEASE • It develops as a consequence of autoimmune destruction of steroidproducing cells in the adrenal gland. • A major autoantigen is 21-hydroxylase (21OH), which is involved in the biosynthesis of cortisol and aldosterone in the adrenal cortex . • Female: Male ratio: 4 : 1 • Susceptibility genes: HLA-DR3 • T cell-mediated injury is likely to be central to pathogenesis. Adrenal autoantibodies may have a pathogenic role, as yet unclear, or could arise secondary to T cell-mediated tissue damage Symptoms & treatments in Addison's disease • • • • • • Weakness Weight loss Poor appetite Hyperpigmentation Hypotension Weak pulses Treatment: Hormone replacement • Cortisol is replaced with a corticosteroid such as hydrocortisone, dexamethasone, or prednisone, and is taken orally one to three times a day. • Aldosterone is replaced with a mineralocorticoid called fludrocortisone acetate taken orally once or twice a day. SKIN AND MUCOSAL MEMBRANES BULLOUS SKIN DISEASES PEMPHIGUS VULGARIS • The most severe and common form of pemphigus • IgG4 autoantibodies against Dsg1 (skin lesion) and Dsg3 (mucosal lesion) • Affects the skin and mucous membranes • Usually begins with painful erosions of the oral mucosa (lasts for several months) • Gradually followed by involvment of the skin • HLA associaton: HLA DR4/14 haplotypes, Dsg3-specific DLA-DR restricted Th2 cells • Patients affected are usually in their fourth to sixth decade of life BULLOUS SKIN DISEASES PEMPHIGUS FOLIACEUS • IgG4 autoantibodies against Dsg1 (EC5 – EC1/EC2 – intramolecular epitope spreading) • Affects skin only, superficial blisters, exfoliative erythroderma • Drug-induced pemphigus: penicillamine Pemphigus foliaceus with large scaly and crusted erosions over the trunk giving a ‘corn flakes’ appearance Pemphigus foliaceus characterised by exfoliative erythroderma DOI: 10.5772/56423 BULLOUS SKIN DISEASES THERAPY • Corticosteroids (prednisone); adjuvant therapy: spearing agents (cyclophosphamide, methotrexate) steroid • The mortality rate has been reduced to less than 10% • Complictions: osteoporosis, diabetes, hypertension, obesity • Patient resistant to steroids: plasmaferesis, IVIG • Rituximab: anti-CD20 mAb Nine months after treatment with rituximab, the patient’s clinical condition remained stable Med J Aust 2008; 189 (5): 289-290. BLOOD DISORDERS AUTOIMMUNE/IDIOPATHIC THROMBOCYTOPENIC PURPURA (A/ITP) Autoimmun condition causing platelet destruction General features • Can be chronic (adults) or acute (children, after acut viral infection) • MHC susceptibility genes are associated with chronic ATP (HLA DRB1*0410) • A variety of infectious diseases are associated with ATP (H. pylori, Hepatitis B,C, HIV) • More common in women than in men (3:1) Pathogenesis: • Specific anti-platelet antibodies targeting platelet membrane glycoproteins Usually IgG, but can be IgM or IgA Cross the placenta, neonatal ATP Antigen: platelet glycoprotein IIb-IIIa or Ib-IX complexes • Autoantibodies bind to platelets resulting in clearance of the opsonized platelets by the phagocytic cells PHAGOCYTE IN ACTION Containing one intact platelet (P) and apparently in the process of phagocytosing another DOI: 10.1056/NEJM197709082971001 AUTOIMMUNE/IDIOPATHIC THROMBOCYTOPENIC PURPURA (A/ITP) Autoimmun condition causing platelet destruction Clinical features: • Thrombocytopenia • Increased numbers of megakaryocytes in the bone marrow • Some patients remain asymptomatic for years • If the number of platelets falls below 109 per liter of blood, severe spontaneous bleeding ensues • Severe mucocutaneous bleeding • Intracranial hemorrhage (rare) THERAPY OF ATP IVIG Saturates Fcγ receptor sites, induces increased expression of the inhibitory receptor FcγRIIB, which can contribute to the inhibition of phagocytosis. The abnormally high level of circulating IgG has a generally suppressive effect on immunoglobulin synthesis. Less severe case: oral prednisone, prednisolone Severe case: dexamethasone, methylprednisolone infusion Remove existing antibodies Not recommended except in an emergency Rituximab: monoclonal anti-CD20 Ab Antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity CD40/CD40L interaction IDEC-131 (mAb to CD40-ligand) Thrombopoietin receptor agonists Romiplostim (sc.): thrombopoiesis stimulating Fc-peptide fusion protein Eltrombopag: orallyadministered agent AUTOIMMUNE HEMOLYTIC ANEMIA (AIHA) Idiopathic AIHA: 50% Warm-reactive antibodies: limphoproliferative diseases, SLE, RA Cold-reactive antibodies: infections (mycoplasma, viral pneumonia, infectious mononucleosis) Drug-induced (methyldopa, penicillin, ceftriaxone) Alloimmune hemolytic anemia Symptoms: • pallor, fatique • shortness of breath, dizziness, headache, • rapid pulse • jaundice, yellowish color of the skin (increased bilirubin) • splenomegaly AUTOIMMUNE HEMOLYTIC ANEMIA (AIHA) Mild cases may not require treatment Treatment: Treat underlying disease, infection Immunosuppressive therapy, corticosteroids Prednisone is thought to decrease monocyte- red cell interactions and decrease autoantibody production. Surgery Prednisone unresponsive patients: splenectomy may be considered. (Pneumococcus vaccine before treatment) Immunotherapy, antibodies IVIG Anti-CD20 (rituximab) Plasmapheresis KIDNEYS AND LUNGS GOODPASTURE’S SYNDROME • Autoantibodies specific for α3 chain of type IV collagen; basement membranes • Autoantibodies are deposited in the basement membranes of organs • High-pressure filtering of blood by renal glomeruli – most sensitive • Glomerulonephritis: IgG is deposited along the basement membranes of renal glomeruli and renal tubules - inflammatory cells accumulate - kidney failure Blood and protein in the urine, high blood pressure, unexplained swelling of limbs or face • Pulmonary hemorrhage: only smokers shortness of breath - coughing up blood, chest pain, • Strong association with HLA-DRB1*15:01/*04 • Therapy: plasmapheresis, immunosuppression (prednisone, cyclophosphamide) MUSCLE CELLS MYASTHENIA GRAVIS Severe muscle weakness ANTAGONISTIC autoantibodies bind to the acetylcholine receptors on muscle cells - receptor endocytosis - degradation The loss of cell-surface acetylcholine receptors makes the muscle less sensitive to neuronal stimulation - progressive muscle weakening Early symptoms: droopy eyelids and double vision • With time, other facial muscles weaken and similar effects on chest muscles impair breathing susceptibility to respiratory infections, can even cause death Therapy: • Pyridostigmine: inhibitor of the enzyme cholinesterase, which degrades acetylcholine- increases the capacity of acetylcholine to compete with the autoantibodies • During crises of severe muscle immunosuppressive drugs (azathioprine) Myasthenia gravis is associated with HLA-DR3 weakening: CENTRAL NERVOUS SYSTEM MULTIPLE SCLEROSIS a4:B1 integrin VCAM Pathogenesis of multiple sclerosis Sclerotic plaques of demyelinated tissue in the white matter of the central nervous system T-cells reencounter antigen on microglial cells (phagocytic macrophage-like cells of the innate immune system resident in the CNS) Inflammation, IFN-γ, IL-17, increased vascular permeability: T -cell, B-cell, macrophage, dendritic cell infiltration, mast cells: histamine Oligoclonal IgG: structural proteins of myelin MULTIPLE SCLEROSIS Pathogenesis of multiple sclerosis • CNS is a relatively immunologically privileged site from which antigens do not normally reach the lymphoid tissues. • In MS, an unknown injurious event is presumed to provoke the release of CNS antigens and their presentation to lymphocytes in the peripheral lymphoid organs. • This results in the expansion of clones of autoreactive T-cells and their differentiation into Th1, Th17 cells, which home to the CNS and initiate inflammation. • Autoantibodies attack the structural proteins of myelin. These include myelin basic protein, proteolipid protein, and myelin oligodendrocyte glycoprotein. MULTIPLE SCLEROSIS • A variety of nervous symptoms: Muscle weakness, impaired vision, ataxia, spasticity (excessive contraction of muscles), paralysis of limbs, urinary incontinence • It can alternate between acute attacks of exacerbating disease and periods of gradual recovery. • The disease is 10 times more frequent in women than in men and is associated with HLA-DR2. • Therapy: Regular subcutaneous injection of IFN-β1 reduces the incidence of disease attacks and the appearance of plaques. Disease attacks: immunosuppressive drugs, corticosteroids