* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The s-Block Elements - GCG-42
Water pollution wikipedia , lookup
De re metallica wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Periodic table wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Atomic theory wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Hydrogen bond wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Electronegativity wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
History of electrochemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Coordination complex wikipedia , lookup
Water splitting wikipedia , lookup
Alkali metal wikipedia , lookup
Electrolysis of water wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Geochemistry wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Liquid-feed flame spray pyrolysis wikipedia , lookup
Ionic liquid wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metallic bonding wikipedia , lookup
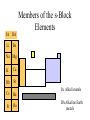
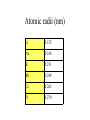
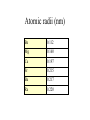
The s-Block Elements Dr. Gurpreet Kaur Members of the s-Block Elements IA IIA Li Be Na Mg K Ca Rb Sr IA Alkali metals Cs Ba Fr Ra IIA Alkaline Earth metals Characteristic properties of alkali metals • • • • • • • • Lowest First I.E & very High Second I.E Soft metals Metallic character Low electronegativity Basic oxides, hydroxides Ionic bond with fixed oxidation state i.e. +1 Characteristic flame colours Weak tendency to from complex Atomic radii (nm) Li 0.152 Na 0.186 K 0.231 Rb 0.244 Cs 0.262 Fr 0.270 Ionization Energies Alkali metals have the lowest I.E in each period and it decreases down the group. Group I 1st I.E. 2nd I.E. Li 519 7300 Na 494 4560 K 418 3070 Rb 402 2370 Cs 376 2420 Electropositive or Metallic character • High tendency to lose eto form positive ions. • Highly electropositive. • Metallic character increases down the group. Characteristic Flame colouration Li Na K Rb Cs HCl(aq) deep red Ca brick red yellow Sr blood red lilac Ba apple green bluish red blue sample Oxides, Peroxide, Superoxide Reaction with water: Oxide: O2- + H2O 2OHPeroxide: O22- + 2H2O H2O2 + 2OHSuperoxide: 2O2- + 2H2O 2OH- + H2O2 + O2 .. .. 2:O:O: .. .. Peroxide ion . .:O:.O: .. .. Super oxide Li does not form peroxide or super oxide Li2O2 Li2O + ½ O2 Basic oxides, hydroxides Oxide Hydroxides Oxide Hydroxides Li2O LiOH BeO Be(OH)2 Na2O, NaOH Na2O2 K2O2, KO2 KOH MgO Mg(OH)2 CaO Ca(OH)2 SrO Sr(OH)2 Rb2O2, RbO2 Cs2O2, CsO2 BaO, Ba2O2 Ba(OH)2 RbOH CsOH Action with hydrogen All the alkali metals react directly with hydrogen to form hydrides which are ionic in nature. 2Na(s) + H2(g) 2NaH(s) The ionic character of hydrides increases from Li to Cs. The stability of hydrides decreases from Li to Cs. The hydrides behave as strong reducing agents and the latter increases from Li to Cs. Action of Halogens All the alkali metals react directly with halogen to produce ionic halides. The reactivity of halogen towards the alkali metal increases down the group. Action with water Alkali metals react with water to form hydroxides and hydrogen gas is evolved. Li reacts slowly but Na and others react violently. That is why they are kept in kerosene oil. All are soluble and basic strength increases down the group. Characteristic properties of alkaline earth metals • • • • • • • Low First I.E & Second I.E Metallic character Low electronegativity Basic oxides, hydroxides Ionic bond with fixed oxidation state i.e. +2 Characteristic flame colours Weak tendency to from complex Atomic radii (nm) Be 0.112 Mg 0.160 Ca 0.197 Sr 0.215 Ba 0.217 Ra 0.220 Ionization Energies Group I 1st I.E. 2nd I.E. 3rd I.E. Be 900 1760 14800 Mg 736 1450 7740 Ca 590 1150 4940 Sr 548 1060 4120 Ba 502 966 3390 1. Have low 1st and 2nd IE. 2. Removal of the 3rd electron is much more difficult as it involves the loss of an inner shell electron. 3. IE decrease as the group is descended. 4. IE of the group II is generally higher than group I. Hydration Enthalpy General trends: 1. On going down both groups, hydration enthalpy decreases. (As the ions get larger, the charge density of the ions decreases, the electrostatic attraction between ions and water molecules gets smaller.) 2. Group 2 ions have hydration enthalpies higher than group 1. ( Group 2 cations are doubly charged and have smaller sizes) Basic oxides, hydroxides Oxide Hydroxides BeO Be(OH)2 MgO Mg(OH)2 CaO Ca(OH)2 SrO Sr(OH)2 BaO, Ba2O2 Ba(OH)2 Hydroxides Group II hydroxide Be Mg Ca Sr Ba Solubility increase, from Amphoteric to basic, base strength increase Variation in Melting Points Strength of metallic bond depends on: 1. Ionic radius 2. Number of e- contributed to the electron sea per atom 3. Crystal lattice structure Note: The exceptionally high m.p. of calcium is due to contribution of d-orbital participation of metallic bonding. Reactions with oxygen Normal Oxide Structure Formed by .. 2:O: .. All alkaline earth metals Peroxide .. .. 2:O-O: .. .. Ba Characteristic Flame colouration Ca brick red Sr blood red Ba apple green HCl(aq) sample Reaction with water M(s) M+(aq) + eH2O(l) + e- OH-(aq) + ½ H2(g) Li Na K Rb Cs -3.05 volt -2.71 -2.93 -2.99 -3.20 Energetic vs. Kinetic Factor Be -1.85 volt Mg -2.38 Ca -2.87 Sr -2.89 Ba -2.90 Action with hydrogen All except Be react directly with hydrogen. Ca(s) + H2(g) CaH2(s) The reactivity increases down the group. Only BeH2 and MgH2 are covalent, others are ionic. Action with halogens BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. Reactions of hydrides They all react readily with water to give the metal hydroxide and hydrogen due to the strong basic property of the hydride ion, H:H:-(s)+ H2O(l) H2(g)+ OH-(aq) Hydride ions are also good reducing agent. They can be used to prepare complex hydrides such as LiAlH4 and NaBH4 which are used to reduce C=O in organic chemistry. Thermal Stability of carbonates Li2CO3 Li2O + CO2 ( at 700oC) All other group I carbonates are stable at ~800oC BeCO3 BeO + CO2 MgCO3 MgO + CO2 CaCO3 CaO + CO2 SrCO3 SrO + CO2 BaCO3 BaO + CO2 ( at 100oC) ( at 540oC) ( at 900oC) ( at 1290oC) ( at 1360oC) Thermal stability 1. Carbonates and hydroxides of Group I metals are as a whole more stable than those of Group II. 2. Thermal stability increases on descending the group. 3. Lithium often follow the pattern of Group II rather than Group I. This is an example of the diagonal relationship. Explanation of Thermal Stability 1. Charge of the ions 2. Size of the ions 3. Compounds are more stable if the charge increases and size decreases. 4. For compounds with large polarizable anions, thermal stability is affected by the polarizing power of the cations. Explanation of Thermal Stability + Decreasing polarizing power - + - + - Increasing stability Relative solubility of Group II hydroxides Compound Solubility / mol per 100g water Mg(OH)2 0.020 x 10-3 Ca(OH)2 1.5 x 10-3 Sr(OH)2 3.4 x 10-3 Ba(OH)2 15 x 10-3 Solubility of hydroxides increases down the group. Solubility of Group II sulphates Compound Solubility / mol per 100g water MgSO4 3600 x 10-4 CaSO4 11 x 10-4 SrSO4 0.62 x 10-4 BaSO4 0.009 x 10-4 Solubility of sulphates increases up the group. Explanation of solubility aqueous M+(aq) + X-(aq) MX(s) H solution H hydration -H lattice M+(g) + X-(g) H solution = -H lattice + H hydration Explanation of solubility 1. Group I compounds are more soluble than Group II because the metal ions have smaller charges and larger sizes. H lattice is smaller, and H solution is more exothermic. H solution = -H lattice + H hydration