* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Immunoexpression of Interleukin 17, Transforming Growth Factor
Survey
Document related concepts
Molecular mimicry wikipedia , lookup
Immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Adaptive immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Innate immune system wikipedia , lookup
Transcript
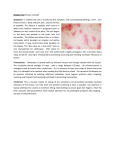
Basic Research—Biology Immunoexpression of Interleukin 17, Transforming Growth Factor b1, and Forkhead Box P3 in Periapical Granulomas, Radicular Cysts, and Residual Radicular Cysts Ana Luiza Dias Leite de Andrade, DDS, MSc,* Cassiano Francisco Weege Nonaka, DDS, MSc, PhD,† ~ ez, DDS, MSc, PhD,‡ Roseana de Almeida Freitas, DDS, MSc, PhD,* Manuel Antonio Gord on-N un and Hebel Cavalcanti Galv~ ao, DDS, MSc, PhD* Abstract Introduction: Different cell types and cytokines have been identified as contributors to the formation of periapical lesions. In this perspective, this study aimed to evaluate the immunoexpression of interleukin (IL)-17, transforming growth factor (TGF)-b1, and the forkhead box P3 (FoxP3) in periapical lesions, correlating them with the type of lesion, the intensity of the inflammatory infiltrate, and the thickness of the cystic epithelial lining. Methods: Twenty periapical granulomas (PGs), 20 radicular cysts (RCs), and 20 residual radicular cysts (RRCs) were submitted to immunohistochemical analysis using anti-IL-17, anti-TGF-b1, and anti-FoxP3 antibodies. Results: In comparison with PGs and RCs, RRCs exhibited a lower immunoexpression of IL-17 and TGF-b1 (P = .021 and P < .001, respectively). The number of FoxP3+ cells increased in this order: RRCs, RCs, and PGs (P < .001). In comparison with lesions with inflammatory infiltrates grades I and II, lesions with inflammatory infiltrate grade III exhibited a higher number of FoxP3+ cells (P = .002). Similarly, in comparison with lesions with inflammatory infiltrates grades II and III, lesions with inflammatory infiltrate grade I showed a tendency for a lower expression of IL-17 and TGF-b1 (P = .085 and P = .051, respectively). For all groups, there was a positive correlation between the immunoexpressions of IL-17 and TGF-b1 (P < .05). Positive correlations between the number of FoxP3+ cells and the immunoexpressions of IL-17 and TGF-b1 (P < .05) were found only in PGs. Conclusions: Th17 and Treg cells seem to interact at the site of injury, suggesting the involvement of proinflammatory and immunoregulatory cytokines in the pathogenesis of periapical lesions. (J Endod 2013;39:990–994) Key Words Cytokines, immunohistochemistry, periapical diseases, regulatory, T lymphocytes, Th17 cells A pical periodontitis is a sequel to endodontic infection and manifests itself as the host defense response to microbial challenge emanating from the root canal system. Periapical granulomas (PGs) consist of a granulomatous tissue with inflammatory cells, fibroblasts, and a well-developed fibrous capsule. If the antigenic stimulus persists, the epithelial cell rests of Malassez can be stimulated by cytokines and growth factors to undergo division and proliferation, an event that may lead to the development of a radicular cyst (RC) (1). Eventually, the tooth affected by an RC is extracted with little regard to the periapical pathosis, which remains within the jaw bone as a residual radicular cyst (RRC) (2). A recent retrospective study in a large case series of radiolucent jaw lesions revealed that PGs and RCs occur at similar rates and together totaled 73% of all lesions. On the other hand, RRCs comprised approximately only 2% of all radiolucent jaw lesions (3). The role of the immune response and bone resorption in the formation of chronic periapical lesions has been extensively studied (4–8). However, little is known about how the set of cytokines involved in the development and maintenance of chronic inflammatory processes is regulated and how the profile of these cytokines is correlated with the composition of the inflammatory infiltrate and clinical presentation of these lesions (5). Th17 and regulatory T (Treg) cells play a critical role in immune cell homeostasis (9). Th17 cells comprise a subpopulation of CD4+ T cells whose main secretion product is interleukin (IL)-17, a proinflammatory cytokine that exerts potent effects on different cell types of the innate immunity and is considered a molecular bridge between the innate and acquired immune systems (10, 11). The forkhead box P3 (FoxP3) transcription factor is the master regulator of Treg cells, which are able to suppress the proliferation and function of different cell types (9). The differentiation of naive T cells into Th17 or Treg lineages is influenced by the level of expression of different cytokines such as transforming growth factor (TGF)b (9, 12). Low levels of TGF-b, IL-23, or IL-6 in the tissue microenvironment induce the development of Th17 cells (13), whereas high levels of TGF-b stimulate the differentiation of Treg cells (14). An imbalance between the differentiation of Th17 and Treg From the *Oral Pathology Postgraduate Program, Department of Dentistry, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil; †Dentistry Postgraduate Program, Department of Dentistry, State University of Paraıba, Campina Grande, Paraıba, Brazil; and ‡Department of Oral Pathology, State University of Paraıba, Paraıba, Brazil. Address requests for reprints to Dr Hebel Cavalcanti Galv~ao, Departamento de Odontologia, Universidade Federal do Rio Grande do Norte, Av Senador Salgado Filho, 1787 Lagoa Nova, Natal, RN, Brazil CEP 59056-000. E-mail address: [email protected] 0099-2399/$ - see front matter Copyright ª 2013 American Association of Endodontists. http://dx.doi.org/10.1016/j.joen.2013.04.028 990 Andrade et al. JOE — Volume 39, Number 8, August 2013 Basic Research—Biology cells causes the loss of host immune homeostasis and the development of autoimmune diseases (8, 9). The objective of the present study was to compare the immunoexpression of IL-17, TGF-b1, and FoxP3 in PGs, RCs, and RRCs and to correlate these findings with the type of lesion, intensity of the inflammatory infiltrate, and thickness of the epithelial lining in the cystic lesions. Materials and Methods Sixty tissue specimens, including 20 PGs, 20 RCs, and 20 RRCs, archived in the files of the Department of Oral Pathology, Federal University of Rio Grande do Norte, Natal, RN, Brazil, were randomly selected for this study. All PGs and RCs were obtained from human teeth without endodontic treatment, and all the RRCs were collected at sites of previous extraction of a tooth affected by a periapical lesion with histopathological diagnosis of RC. The study was approved by the Ethics Committee of the Federal University of Rio Grande do Norte (protocol number: 132/11-P). Morphologic Analysis For the morphologic analysis, 5-mm-thick tissue sections were stained with hematoxylin-eosin. The intensity of the inflammatory infiltrate was evaluated according to the method adapted from Tsai et al (15). The grading of each specimen was recorded on the inflammatory condition in 3 consecutive microscopic fields, starting from the inner portion of the specimen and proceeding deeper into connective tissue. Briefly, each specimen was graded at 400 magnification as follows: grade I, inflammatory cells restricted to the first microscopic field; grade II, inflammatory cells extending to the second microscopic field; and grade III, inflammatory cells in all 3 microscopic fields. The thickness of the epithelial lining was defined as atrophic (2–10 cell layers and flat epithelial/capsule boundary) or hyperplastic (>10 cell layers and undulating epithelial/capsule boundary often arranged into proliferating arcades) (16) based on the predominant pattern in each case. Immunohistochemical Methods For the immunohistochemical study, 3-mm-thick sections were obtained from paraffin-embedded tissue blocks, deparaffinized, and immersed in 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were then washed in phosphate-buffered saline (PBS) and submitted to antigen retrieval (Table 1). After treatment with normal serum, the sections were incubated with the primary anti–IL-17, anti–TGF-b1, and anti-FoxP3 antibodies (Table 1) in a moist chamber. The sections were then washed twice in PBS and incubated at room temperature with a 2-step polymer-based complex (Advance HRP; Dako, Carpinteria, CA) for anti–IL-17 and anti–TGFb1 antibodies and with a 1-step polymer-based complex (EnVision Dual Link System-HRP, Dako) for anti-FoxP3 antibody. Peroxidase activity was visualized by immersing the tissue sections in diaminobenzidine (Liquid DAB+ Substrate, Dako), which resulted in a brown reaction product. Finally, the sections were counterstained with Mayer hematoxylin and coverslipped. Sections of human normal tonsil were used as positive controls for IL-17 and FoxP3. Mouse uterus extract served as positive controls for TGF-b1. As negative controls, the samples were treated as described previously except that the primary antibody was replaced with a solution of bovine serum albumin in PBS. Immunostaining Assessment and Statistical Analysis Immunohistochemical analysis was performed in a blind fashion by 2 observers under an Olympus CX41 light microscope (Olympus Japan Co, Tokyo, Japan). Tissue sections were examined by light microscopy (100 magnification) to identify 5 fields with the largest number of immunostained cells. Digital images of these 5 microscopic fields (400 magnification) were acquired using an Olympus EVOLT E330 digital camera (Olympus Japan Co) and loaded on the software Image J (National Institute of Mental Health, Bethesda, MD). With respect to IL-17 and TGF-b1, the number of positive and negative cells was determined in each field, permitting the calculation of the percentage of IL-17- and TGF-b1–positive cells in each case. FoxP3+ cells were counted, and the total number of positive cells per case was calculated, permitting the calculation of the mean number of FoxP3+ cells per specimen. The results obtained were submitted to statistical analysis using the Statistical Package for the Social Sciences (version 17.0; SPSS Inc, Chicago, IL). After analysis for normality and variance of the data, the nonparametric Mann-Whitney U and Kruskal-Wallis tests were applied. The Spearman correlation test was performed to determine possible correlations. For all tests, significance level was set at 5% (P < .05). Results Morphologic Analysis Analysis of the inflammatory infiltrate in PGs revealed 18 cases (90.0%) with inflammatory infiltrate grade III and 2 cases (10.0%) with grade II. In RCs, 13 cases (65.0%) showed inflammatory infiltrate grade III and 7 cases (35.0%) grade II. In RRCs, 10 cases (50.0%) showed inflammatory infiltrate grade II, 6 cases (30.0%) grade III, and 4 cases (20.0%) grade I. Morphologic analysis of the epithelial thickness in RCs revealed the presence of an atrophic epithelium in 10 cases (50.0%) and a hyperplastic epithelium in 10 cases (50.0%). In RRCs, 14 cases (70.0%) presented an atrophic epithelium, and 6 cases (30.0%) showed a hyperplastic epithelium. Immunohistochemical Analysis Immunoexpression of IL-17. Analysis of the immunoexpression was positive for all evaluated cases of PGs, RCs, and RRCs (Fig. 1A– C). The median percentage of IL-17–positive cells was 69.85 in PGs, 70.20 in RCs, and 52.55 in RRCs (Table 2). A statistically significant higher median percentage of IL-17–positive cells was identified in PGs and in RCs when compared with RRCs (P = .021) (Table 2). With respect to the intensity of the inflammatory infiltrate, the median percentage of IL-17–positive cells was 50.40 in lesions with inflammatory infiltrate grade I, 69.80 in lesions with grade II, and TABLE 1. Manufacturer, Clone, Antigen Retrieval, Dilution, and Incubation Period of the Primary Antibodies Antibody IL-17 TGF-b1 FoxP3 Manufacturer Clone Antigen retrieval Dilution Incubation Santa Cruz Biotechnology Santa Cruz Biotechnology Santa Cruz Biotechnology H-132 V H-190 Citrate, pH 6.0, Pascal, 121 C, 3 min Citrate, pH 6.0, Pascal, 121 C, 3 min Citrate, pH 6.0, Pascal, 121 C, 3 min 1:100 1:700 1:150 60 min Overnight Overnight JOE — Volume 39, Number 8, August 2013 IL-17, TGF-b1, and FoxP3 991 Basic Research—Biology Figure 1. Representative photomicrographs of immunohistochemical staining of the studied markers in periapical lesions. (A–C) The immunohistochemical expression of IL-17 in PGs, RCs, and RRCs, respectively. (D–F) The immunohistochemical expression of TGF-b in PGs, RCs, and RRCs, respectively. (G–I) The immunohistochemical expression of FoxP3 in PGs, RCs, and RRCs, respectively. 66.50 in lesions with grade III. No statistically significant differences was observed between the groups (P = .085) (Table 2). Considering the epithelial thickness, the median percentage of IL17–positive cells was 62.15 in lesions with hyperplastic epithelium and 65.90 in lesions with atrophic epithelium. No statistically significant differences was verified between the groups (P = .868) (Table 2). Immunoexpression of TGF-b1. Analysis of the immunoexpression of TGF-b1 showed positivity for all cases of PGs and RCs and in TABLE 2. The Mean, the Standard Error of the Mean (SEM), and the Median Number of IL-17–, TGF-b–, and FoxP3-positive Cells and Their Differences in Relation to the Type of Lesion, the Intensity of the Inflammatory Infiltrate, and Epithelial Thickness Immunoexpression of IL-17–positive cells Variable Type of lesion PG RC RRC Inflammatory infiltrate Grade I Grade II Grade III Epithelial thickness Hyperplastic epithelium Atrophic epithelium Immunoexpression of TGF-b–positive cells Immunoexpression of FoxP3-positive cells P Mean ± SEM Median P 69.85 70.20 52.55 .021* 62.06 3.82 54.82 2.91 34.38 3.56 64.65 56.85 35.70 <.001* 3.05 0.55 1.36 0.26 0.33 0.07 2.10 1.10 0.20 <.001* 51.37 7.68 69.38 3.14 63.90 2.29 50.40 69.80 66.50 .085* 23.55 10.91 50.84 3.99 53.11 2.98 20.70 52.70 56.00 .051* 0.10 0.05 0.91 0.25 2.08 0.36 0.10 0.60 1.40 .002* 63.80 3.11 62.05 3.65 62.15 65.90 .868† 45.89 4.57 43.74 3.60 49.00 46.10 .847† 1.28 0.34 0.55 0.10 0.90 0.40 .066† n Mean ± SEM Median 20 20 20 68.91 2.32 67.78 3.60 57.71 3.14 4 19 37 16 24 Mean ± SEM Median P *Kruskal-Wallis test. † Mann-Whitney U test. 992 Andrade et al. JOE — Volume 39, Number 8, August 2013 Basic Research—Biology TABLE 3. The Spearman Correlation Test between the Number of FoxP3+ Cells and the Percentage of IL-17– and TGF-b–positive Cells According to the Type of Lesion Type of lesion PGs FoxP3 IL-17 FoxP3 TGF-b IL-17 TGF-b RCs FoxP3 IL-17 FoxP3 TGF-b IL-17 TGF-b RRCs FoxP3 IL-17 FoxP3 TGF-b IL-17 TGF-b n r P 20 20 20 0.451 0.590 0.484 .046 .006 .031 20 20 20 0.242 0.093 0.657 .303 .697 .002 20 20 20 0.120 0.072 0.709 .615 .762 <.001 19 RRCs (95.0%) (Fig. 1D–F). The median percentage of TGF-b1– positive cells was 64.65 in PGs, 56.85 in RCs, and 35.70 in RRCs (Table 2). A statistically significant higher median percentage of TGFb1–positive cells was identified in PGs and in RCs when compared with RRCs (P < .001) (Table 2). In relation to the inflammatory infiltrate, the median percentage of TGF-b1–positive cells was 20.70 in lesions with inflammatory infiltrate grade I, 52.70 in lesions with grade II, and 56.00 in lesions with grade III. A higher median percentage of TGF-b1–positive cells was verified in lesions with inflammatory infiltrates grade II and III when compared with those with grade I. However, the P value indicated borderline significance (P = .051) (Table 2). Regarding the epithelial thickness, the median percentage of TGFb1–positive cells was 49.00 in lesions with hyperplastic epithelium and 46.10 in lesions with atrophic epithelium. No statistically significant differences was observed between the groups (P = .847) (Table 2). FoxP3+ Cells. Analysis of the immunoexpression of FoxP3 showed the presence of FoxP3+ cells in 19 PGs, 19 RCs, and 14 RRCs (Fig. 1G– I). The median number of FoxP3+ cells was 2.10 in PGs, 1.10 in RCs, and 0.20 in RRCs (Table 2). A statistically significant higher median number of FoxP3+ cells was identified in PGs when compared with RCs and RRCs (P < .001) (Table 2). Considering the intensity of the inflammatory infiltrate, the median number of FoxP3+ cells was 0.10 in lesions with inflammatory infiltrate grade I, 0.60 in lesions with grade II, and 1.40 in lesions with grade III. A statistically significant higher median of FoxP3+ cells was observed in lesions with inflammatory infiltrate grade III when compared with those with inflammatory infiltrate grades I and II (P = .002) (Table 2). In relation to the epithelial thickness, the median number of FoxP3+ cells was 0.90 in lesions with hyperplastic epithelium and 0.40 in lesions with atrophic epithelium. No statistically significant differences were verified between the groups (P = .066) (Table 2). The Spearman correlation test disclosed moderate positive correlations between the number of FoxP3+ cells and the percentage of IL17– and TGF-b1–positive cells in PGs (Table 3). In RCs, a moderate positive correlation was observed between the percentages of IL-17– and TGF-b1–positive cells. Similarly, a strong positive correlation was observed between the percentages of IL-17– and TGF-b1–positive cells in RRCs. Discussion Inflammatory periapical lesions are characterized by the infiltration of different immune cells in the periapical tissues as well as the production of inflammatory mediators such as cytokines (4, 8). JOE — Volume 39, Number 8, August 2013 Among the different cells that infiltrate the periradicular tissues in an attempt to eradicate the infection, studies have highlighted the role of T lymphocytes through Th1, Th2, Th17, and Treg responses (8, 17– 19). The present study evaluated the immunoexpression of IL-17 and TGF-b1 in PGs, RCs, and RRCs. Analysis of the immunoexpression of IL-17 showed a smaller number of positive cells in RRCs compared with PGs and RCs (P = .021). Our results disagree with Marçal et al (8) who observed higher immunoexpression of IL-17 in cases of PGs compared with RCs (Table 2). IL-17 induces inflammatory responses, contributes to the development of Th1 immunity, and stimulates osteoclastic bone resorption in combination with receptor activator of nuclear factor kappa B and its ligand (7, 20). It is speculated that IL-17 exerts protective effects and is also involved in bone destruction during the onset and development of periapical lesions. This cytokine may be one of the factors stimulating the expression of inflammatory effectors, with most of them acting on bone metabolism by promoting osteoclastogenesis. In addition, IL-17 participates in the recruitment and activation of neutrophils and protects against infection-induced alveolar bone loss in periapical lesions (5, 7, 8, 20). In this perspective, the observation of a larger mean number of IL17–positive cells, a proinflammatory cytokine, in PGs and RCs may be caused by the constant stimulation by bacterial toxins released into the infected root canal and the higher proliferative activity of these lesions. The lower immunoexpression of IL-17 in RRCs suggests that, although histopathologically similar, RCs and RRCs possess different biochemical properties because the main antigen stimulus is absent in the latter (21). With respect to TGF-b, this potent cytokine mediates regulatory functions and exerts variables effects on hematopoietic cells (22). In addition, TGF-b is believed to play a critical role in the development, maintenance, and induction of Treg and Th17 cells (9, 12, 23). The analysis of the immunoexpression of TGF-b1 showed significantly higher immunoreactivity in PGs and RCs when compared with RRCs (P < .001). A similar expression of TGF-b1 in PGs and RCs has also been reported in previous studies (8, 24, 25). However, Fukada et al (26) reported the absence of differences, and Garlet et al (27) showed that TGF-b1 expression was significantly higher in the inactive PGs. The reason why TGF-b levels are elevated in periapical lesions is still not clear, but it seems to be associated with the presence of large numbers of cells producing this cytokine, including macrophages, fibroblasts, eosinophils, osteoclasts, osteoblasts, and Treg cells (24, 25, 28). TGF-b has been suggested to be important for periapical healing because this cytokine is known to inhibit bone resorption activity and to promote bone remodeling and tissue repair. In this sense, studies indicated that TGF-b stimulates collagen synthesis, neovascularization, and fibroblast proliferation (24). According to Santos et al (29), the intensity of the inflammatory infiltrate is not only related to an increase in the amount of antigen stimuli but also to an exacerbated response of defense mechanisms of the organism. So, the results of this study confirm the possible role of TGF-b1 in the stabilization of periapical lesions through its immunosuppressive and regulatory effects on inflammation (28), with the observation of higher immunostaining for this cytokine in more inflamed lesions (P = .051). In the present study, the mean number of FoxP3-immunopositive cells was significantly higher in PGs followed by RCs and RRCs (P < .001). These results agree with Fukada et al (26) and Peixoto et al (30) who also observed a higher expression of FoxP3 in cases of PGs when compared with RCs. On the other hand, Marçal et al (8) found differences in the immunoexpression of FoxP3 only when PGs and RCs were compared with the control group but not between lesions. IL-17, TGF-b1, and FoxP3 993 Basic Research—Biology Previous studies have confirmed the presence of Treg cells in periapical lesions, and it was postulated that these cells infiltrate such lesions to inhibit the proliferation of T cells (26, 31, 32). In addition, although the mechanisms underlying the development of human periapical lesions are not fully understood, Treg cells seem to critically regulate the imbalance in the activity of osteoclastic and immune cells in PGs and RCs (26). The increase in the number of positive lymphocytes from RCCs to RCs and from RCs to PGs might be proportional to the pathobiological activity of these periapical lesions. A positive correlation was observed between the immunoexpression of FoxP3 and the intensity of the inflammatory infiltrate (P = .002). As observed for TGF-b1 immunoreactivity, Treg cells may exert their regulatory functions by controlling the immune and inflammatory processes seen in periapical lesions. Hence, a larger number of this cell type would be present in more intensely inflamed lesions in an attempt to regulate the immunologic mechanisms triggered in these lesions (32). Although statistical significant differences were not observed (P > .05), a larger mean number of FoxP3-positive cells was verified in cystic lesions with hyperplastic epithelium. According to Moreira et al (16), despite the presence of antigens able to induce immune responses, epithelial proliferation, and bone resorption, no cystic growth is observed in lesions with atrophic epithelium. Therefore, immunosuppressive effector molecules or apoptotic events may act during these stages, regulating cystic growth (16, 33). In this perspective, the increased proliferative activity is influenced by the presence of inflammation at different levels in the capsule, whereas cysts lined by hyperplastic epithelium are more inflamed than those covered by atrophic epithelium (34). In agreement with other studies (19, 32, 35), a positive correlation between the immunoexpression of the markers and inflammatory periapical lesions was also shown in the present study. As a consequence, the correlation between the immunoexpression of IL-17 and TGF-b would be caused by the participation of proinflammatory and immunoregulatory cytokines in the pathogenesis of periapical diseases (19). The present results highlight the complexity of the mechanisms underlying the immunopathogenesis of periapical lesions. A wide variety of immune responses are triggered in periradicular tissues, which leads to the establishment of different cell interactions. Th17 and Treg cells participate in this process, exerting opposite modulatory effects. Nevertheless, these cells can be present simultaneously. Acknowledgments The authors deny any conflicts of interest related to this study. References 1. Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 2004;15:348–81. 2. Dimitroulis G, Curtin J. Massive residual dental cyst: case report. Aust Dent J 1998; 43:234–7. 3. Koivisto T, Bowles WR, Rohrer M. Frequency and distribution of radiolucent jaw lesions: a retrospective analysis of 9,723 cases. J Endod 2012;38:729–32. 4. Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med 1998;9:498–521. 5. Colic M, Vasilijic S, Gazivoda D, et al. Interleukin-17 plays a role in exacerbation of inflammation within chronic periapical lesions. Eur J Oral Sci 2007;115: 315–20. 6. Menezes R, Garlet TP, Letra A, et al. Differential patterns of RANKL/OPG expression in human periapical granulomas: possible association with progressive or stable nature of the lesions. J Endod 2008;34:932–8. 994 Andrade et al. 7. Oseko F, Yamamoto T, Akamatsu Y, et al. IL-17 is involved in bone resorption in mouse periapical lesions. Microbiol Immunol 2009;53:287–94. 8. Marçal JR, Samuel RO, Fernandes D, et al. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod 2010;36:995–9. 9. Chen Z, Lin F, Gao Y, et al. FOXP3 and RORgt: transcriptional regulation of Treg and Th17. Int Immunopharmacol 2011;11:536–42. 10. Gaffen SL, Kramer JM, Yu JJ, et al. The IL-17 cytokine family. Vitam Horm 2006;74: 255–82. 11. Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci 2008;13:170–7. 12. Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem 2010;147:781–92. 13. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009;30:646–55. 14. Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008;453: 236–40. 15. Tsai CH, Weng SF, Yang LC, et al. Immunohistochemical localization of tissue-type plasminogen activator and type I plasminogen activator inhibitor in radicular cysts. J Oral Pathol Med 2004;33:156–61. 16. Moreira PR, Santos DF, Martins RD, et al. CD57+ cells in radicular cyst. Int Endod J 2000;33:99–102. 17. Gazivoda D, Dzopalic T, Bozic B, et al. Production of proinflammatory and immunoregulatory cytokines by inflammatory cells from periapical lesions in culture. J Oral Pathol Med 2009;38:605–11. 18. Queiroz-Junior CM, Silva MJ, Corr^ea JD, et al. A controversial role for IL-12 in immune response and bone resorption at apical periodontal sites. Clin Dev Immunol 2010;2010:327417. 19. Colic M, Gazivoda D, Vucevic D, et al. Proinflammatory and immunoregulatory mechanisms in periapical lesions. Mol Immunol 2009;47:101–13. 20. Xiong H, Wei L, Peng B. Immunohistochemical localization of IL-17 in induced rat periapical lesions. J Endod 2009;35:216–20. 21. Muglali M, Komerik N, Bulut E, et al. Cytokine and chemokine levels in radicular and residual cyst fluids. J Oral Pathol Med 2008;37:185–9. 22. Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146. 23. Romagnani S. Regulation of the T cell response. Clin Exp Allergy 2006;36:1357–66. 24. Tyler LW, Matossian K, Todd R, et al. Eosinophil-derived transforming growth factors (TGF-alpha and TGF-beta1) in human periradicular lesions. J Endod 1999;25:619–24. 25. Teixeira-Salum TB, Rodrigues DB, Gervasio AM, et al. Distinct Th1, Th2 and Treg cytokines balance in chronic periapical granulomas and radicular cysts. J Oral Pathol Med 2010;39:250–6. 26. Fukada SY, Silva TA, Garlet GP, et al. Factors involved in the T helper type 1 and type 2 cell commitment and osteoclast regulation in inflammatory apical diseases. Oral Microbiol Immunol 2009;24:25–31. 27. Garlet GP, Horwat R, Ray HL Jr, et al. Expression analysis of wound healing genes in human periapical granulomas of progressive and stable nature. J Endod 2012;38: 185–90. 28. Danin J, Linder LE, Lundqvist G, et al. Tumor necrosis factor-alpha and transforming growth factor-beta1 in chronic periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:514–7. 29. Santos LC, Ramos EA, Gurgel CA, et al. Immunohistochemical detection of Langerhans cells in dental granulomas and radicular cysts. J Mol Histol 2007;38: 201–5. 30. Peixoto RF, Pereira Jdos S, Nonaka CF, et al. Immunohistochemical analysis of FoxP3+ cells in periapical granulomas and radicular cysts. Arch Oral Biol 2012; 57:1159–64. 31. Alshwaimi E, Purcell P, Kawai T, et al. Regulatory T cells in mouse periapical lesions. J Endod 2009;35:1229–33. 32. Colic M, Gazivoda D, Vucevic D, et al. Regulatory T-cells in periapical lesions. J Dent Res 2009;88:997–1002. 33. Loyola AM, Cardoso SV, Lisa GS, et al. Apoptosis in epithelial cells of apical radicular cysts. Int Endod J 2005;38:465–9. 34. Martins CA, Rivero ER, Dufloth RM, et al. Immunohistochemical detection of factors related to cellular proliferation and apoptosis in radicular and dentigerous cysts. J Endod 2011;37:36–9. 35. Nakajima T, Ueki-Maruyama K, Oda T, et al. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res 2005;84:639–43. JOE — Volume 39, Number 8, August 2013