* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Immunology of the tonsil: a review
Survey
Document related concepts
Monoclonal antibody wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adaptive immune system wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Transcript
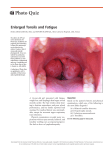
104 Journal of the Royal Society of Medicine Volume 83 February 1990 Immunology of the tonsil: Glenis K Scadding MD MRCP a review Royal National Throat, Nose and Ear Hospital, Grays Inn Road WC1 Keywords: tonsils; tonsillectomy; mucosal associated lymphoid tissues (MALT); secretory immunoglobulins; B lymphocytes Despite the fact that tonsils have been removed for several hundred years knowledge of their exact function and of the effects of their removal is remarkably incomplete. The tonsils undoubtedly form part of the immune system, participating in its function of recognition and rejection of foreign materials and organisms. Immune system Innate non-specific immunity involves physical and chemical barriers, phagocytic cells and humoral factors, such as complement and interferon. Superimposed on this primitive system in vertebrates is adaptive immunity, where the response, which is mediated by lymphocytes and antibodies, improves on second and subsequent contact with the same organism, ie it possesses both specificity and memory. Lymphoid system Lymphocytes are organized into primary (bone marrow and thymus) and secondary (spleen, lymph nodes and mucosal-associated) lymphoid tissues (Figure 1). The bone marrow is the site of origin of lymphocytes: T cells migrate from there to the thymus where they undergo further 'education', becoming able to recognize antigens only in the context of self histocompatibility antigens, before becoming widespread throughout the blood and lymphoid system. B cells pass from marrow to the secondary lymphoid organs and blood where they make contact with antigen and with other lymphocytes. The immune response is generated and disseminated from the secondary lymphoid organs. Figure 1. Section ofhuman tonsil showing MALT. This view shows the large number ofgerminal centres frequently found in tonsillar lymphoid tissue (reprinted with kind permission of Mr C Symes and Gower Medical, London from Roitt, Brostoff Male, eds. Immunology, 2nd edn, 1989) The mucosal associated lymphoid tissues (MALT) are thought of as a special type of secondary lymphoid organ, concerned with immunity at mucosal surfaces. The MALT includes Peyer's patches, appendix, and other gut lymphocytes, known collectively as gutassociated lymphoid tissue (GALT), together with bronchial-associated lymphoid tissue (BALT), adenoid, oral, palatinal, lingual and laryngeal tonsils. The major function of the MALT is the generation and dissemination of antigen-sensitized B cells which require second signals for their terminal differentiation to antibody-producing plasma cells in various secretory tissues. Paper read to Section of Laryngology, 3 March 1989 The tonsils The tonsils are placed at the gateway of the respiratory and alimentary tracts where they are continually directly bombarded by antigen, unlike the majority of lymph nodes, which receive antigen via the bloodstream. At the base of the tonsillar crypts are specialized micropore (M) cells with a tubulovesicular system for antigen transport'. In addition there are micropores found on the crypt wall, as yet it is not known whether these are artefactual, physiological or pathological2. By analogy with events known to occur in other parts of the MALT3, the tonsils probably function in the following way. Within the tonsil antigen is taken up by antigenprocessing cells, which are of the macrophage lineage, and presented to T helper cells and B cells. These latter form the major constituent of the many germinal centres present in tonsil. (Figures 1 and 2). Under the correct conditions those B cells bearing Figure 2. Secondary lymphoid follicle section showing a germinal centre. The germinal centre contains actively proliferating B cells. The darker half contains the most actively proliferating cells, together with macrophages. There is a mantle of small resting lymphocytes, which have less cytoplasm and appear more densely packed (reprinted with kind permission of Dr K McLennan and Gower Medical, London, from Roitt, Brostoff Male, eds. Immunology, 2nd edn, 1989) 0141-0768/90/ 020104-04402.00/0 1990 The Royal Society of Medicine Journal of the Royal Society of Medicine Volume 83 February 1990 receptors, ie antibodies, capable of combining with determinants on the antigen will be stimulated to divide. They then migrate through lymph and blood, undergoing further differentiation en route, to colonize various secretory structures,- ,such as gut, respiratory tract, salivary and mammary glads. Here they are subjected to 'second signals' which are only partly defined, but could include antigen, T cell factors, mitogens or local hormones. These cause the terminal differentiation of the lymphocytes to immunoglobulin-secreting plasma cells. The sites to which lymphocytes 'home' seems to depend to some extent upon site of origin. There is evidence to suggest that gut-derived lymphocytes home selectively to gut, those from the tonsil probably return to the upper and lower respiratory tract, including tonsil. Salivary and mammary glands appear to derive lymphocytes from both sources4. After this homing process there is still lymphocyte traffic between the various lymphoid organs via lymphatics and blood, with about 1-2% of the lymphocyte pool recirculating each hour. This allows lymphocytes to come into contact with their appropriate antigen, an important process since there is a finite number of lymphocytes capable of recognizing any one antigenic determinant. The majority of MALT lymphocytes secrete immuno globulin A (IgA) which acts as a 'mucosal antiseptic paint'. It exists as a dimer, the two molecules being joined by a J chain, also secreted by the plasma cell. Dimeric IgA passes through epithelial cells to reach the mucosal surface, during this process it becomes coated with secretory piece which- protects the molecule from enzymic digestion (Figure 3). IgA combines with pathogens or other molecules, either preventing their attachment. and absorption or rendering them harmless, so that they are absorbed, transported as an immune complex and dealt with by the reticulo-endothelial system. Tonsillar imunoglobulin secretion differs from the usual MALT pattern. Immunoglobulin G-producing cells predominate in palatine and nasopharyngeal Figure 3. Transport of IgA across the mucosal epithelium. IgA dimers secreted by plasma cells bind to membrane receptors on the internal surface ofepithelial cells. They are endocytosed and transported across the cell to the luminal surface where the vesicle fuses with the plasma membrane, releasing dimeric IgA and a secretory component derived from cleavage of the receptor. This probably protects the immunoglobulin from enzymic digestion (reprinted with kind permission of Gower Medical, London from Roitt, Brostoff Male, eds. Immunology, 2nd edn, 1989) Figure 4. Natural killer cell in human tonsil. Two natural killer cells, stained with monoclonal antibody HNK-1 conjugated with horseradish peroxidase, are seen close to blood vessels. These cells, which form part of the innate immune system, can kill cells with surface alterations due to viral infection or neoplasia (courtesy Ms H Wang) tonsils5, with IgA immunocytes representing about 30-35%. Unlike the adenoids there is -no tonsillar production of secretory piece, so both IgA and IgG pass out directly into the pharyngeal secretions by leaking between epithelial cells, this is enhanced when there is inflammation. IgD-producing cells are far more plentiful than in GALT, although they represent only 1-3% of the tonsillar immunocyte population6. IgEproducing cells can also be found in tonsil, occurring more frequently in tonsillectomy specimens than does atopy in the population, suggesting that allergic children are more likely to come to tonsillectomy. These differences probably reflect an adaptation to the unique situation of the tonsil. Bacteria that inhabit the upper respiratory tract (but not those in gut) have receptors for IgD, these probably- stimulate IgD-bearing B cells to divide, hence increasing the numbers present in tonsil7. Immunological functions other than antibody production A tonsil contains up to 109 lymphoid cells, up to 50% of which are T cells. Many of these will be involved in the regulation of the antibody response, either promoting it (helper T cells) or preventing it (suppressor T cells). Other T cells are responsible for delayed type hypersensitivity reactions to large organisms, such as fungi. Another type can kill virally infected cells. Recognition in both cases is by the T cell antigen receptor, which is similar to the antigen combining site of antibody. Cytokines, such as interferon gamma, are produced by tonsillar T cells. Natural killer cells are also present in tonsil, closely apposed to blood vessels (Figure 4). These form part of the innate immune system and can kill virusinfected and tumour cells, but their method of recognizing such cells is as yet unknown. Immune deficiency Selective deficiency of IgA is the commonest humoral immune deficiency, affecting about 1 in 700 of the population. In children IgA deficiency is often transient, levels increasing to normal by about 7 years. There is an association between this and allergy. In some patients with selective IgA deficiency secretory IgA is lacking, but may be replaced by secretory IgM, which is also protective. In others 105 106 Journal of the Royal Society of Medicine Volume 83 February 1990 immunoregulatory compensation gives rise to a large number of IgD-producing cells in respiratory mucosa. This cannot act as secretory antibody, hence these individuals tend to have recurrent respiratory tract infections8. IgD-bearing cells are increased in tonsillectomy specimens. Inflammation of the tonsils Antigens are continuously present on the crypt epithelium giving rise to lymphocyte activation, thus a certain amount of inflammation is physiological. Recurrent or chronic inflammation Histologically, chronically inflamed tonsils differ little from 'normal' ones, which have been relatively little studied. There is a decrease in activated lymphoid celLs and immiunoglobulin containing cells9, which could be primary, or the result of infections. The relative increase in IgD-bearing cells as noted earlier may be due to bacterial stimulation. Epithelial changes also occur, with an initial increase in reticulated epithelium to cover the interfollicular areas as well as the follicles. Later the follicles become covered in squamous epithelium, which lacks M cells, thus antigen entry is likely to be reduced10. Tonsillitis occurs when trapped organisms multiply within and on the tonsil. Such infections are frequently polymicrobial. There are likely to be predisposing factors to this, including failure of host defence and virulence of the organism itself. Local production of B-lactamase by other bacteria within the tonsil has been shown to occur11. This can prevent penicillin antibiotics from destroying otherwise sensitive organisms. Bacteria can also release histamine from tonsillar mast cells'2, contributing to the inflammation. Viral infection is itself a cause of tonsillar inflammation; chronic infection with Epstein-Barr virus also reduces host resistance to other organisms by stimulating the production of T suppressor cells. It is conceivable that some episodes of upper respiratory tract infection and tonsillitis are allergic rather than infective in origin. It is now known that the late phase of the allergic reaction, which involves infiltration by inflammatory cells, mucosal oedema and hyperreactivity, can last from hours to weeks following allergen contact, and is probably responsible for much of the morbidity associated with allergy13. With chronic exposure to allergens such as house dust mite or animal dander the initial immediate reaction is not always apparent, so that careful history taking and skin prick testing are necessary. Symptoms may be caused by allergy alone, or by infection subsequent to mucosal oedema and blockage. Treatment of the underlying allergy should reduce the frequency of episodes of 'tonsillitis'. Tonsillectomy The human tonsils are most active in childhood, with some involution after puberty. However considerable B-cell activity is seen in clinically healthy adult tonsils, even at 80 years of age. The indications for, and the therapeutic effects of, tonsillectomy for recurrent infections remain the subject of debate. There is no evidence for benefit from the operation in preventing the recurrence of streptococcal hypersensitivity disorders, such as rheumatic fever. The practice of tonsillectomy and/or adenoidectomy has decreased over the past decade, since the operations have little influence on the natural history of upper respiratory tract infections. A recent unique controlled studyl4 involving 12 authors over 11 years showed that the benefit from tonsillectomy in patients selected on rigorous criteria with recurrent severe tonsillitis was modest. The advantage of the operated over the non-operated group was present for two years, but most of the non-operated group had less than three infections a year, mostly mild, compared with a 14% incidence of surgery-related complications. A similar earlier study15 showed a decrease in sore throats for the first two postoperative years only, probably insufficient benefit to justify the risks of operation. Children with perennial allergic rhinitis were found in a recent study'6 to be four times more likely to have undergone an ENT operation than those with orthopaedic problems. Only 40% improved following operation, whereas 90% improved with medical treatment of their allergies. Immunological effects of tonsillectomy These have not been extensively studied and investigations are hampered by the fact that any immune defect could have been present preoperatively, since sequential studies are few. However, there is evidence for a decrease in the activated B cell population, with a decrease in secretory IgA and a fall in other immunoglobulins, but only to the lower end of the normal range'7. There is no evidence of a general increase in infections postoperatively compared to an age-matched control group. Resistance to polioviruses is however reduced'8. Children who were previously immunized orally with live poliovirus vaccine dropped their titres three-fold to four-fold after tonsillectomy and adenoidectomy. Attempts to vaccinate seronegative children who had been subjected to tonsillectomy and adenoidectomy resulted in delayed and lowered nasopharyngeal secretory immune responses as measured by IgA antibodies to poliovirus. It was thought that the increased meningococcal carrier state after tonsillectomy could increase the risk of meningococcal meningitis several months later. However this has occurred only in Russia (five reports since 1959). An increase in Hodgkin's disease in tonsillectomized children in New York was reported in 197119 Since then several other studies have investigated this with conflicting results20. In fact the association could be with tonsillitis, rather than tonsillectomy. Although the effects reported are slight the role of Waldeyer's ring in reinforcing immunity of the whole upper respiratory tract should be considered before a child is subjected to tonsillectomy, as well as the possibility that some underlying disorder such as allergy or IgA deficiency is responsible for his or her symptoms. References 1 Olah I, Surjan L, Jr, Toro I. Electronmicroscopic observations on the antigen reception in the tonsillar tissue. Acta Acad Sci Biol Hung 1972;23:61-73 2 Howie AJ. Scanning and transmission electronmicroscopy of the epithelium of human palatine tonsils. J Pathol 1980;130:91-8 3 Elson CO. Induction and control of the gastrointestinal immune system. Scand J Gasfrenti3rol 1985;2(KSuppl 14kl1 Journal of the Royal Society of Medicine Volume 83 February 1990 4 Brandtzaeg P. Immunobarriers of the mucosa of the upper respiratory and upper digestive pathways. Acta Otolaryngol (Stockh) 1988;105:172-80 5 Brandtzaeg P, Surgan L, Berdal P. Immunoglobulin system of human tonsils. 1. Control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry, and serum Ig concentrations. Clin Exp Immunol 1978;31:367-75 6 Korsrud FR, Brandtzaeg P. Immune system of human nasopharyngeal and palatine tonsils: histomorphometry of lymphoid components and quantification of immunoglobulin-producing cells in health and disease. Clin Exp Immunol 1980;39:361-70 7 Forsgren A, Grubb AO. Many bacterial species bind human IgD. J Immunol 1979;122:1468-72 8 Brandtzaeg P, Karlsson G, Petruson B, Bjorkander J, Hanson LA. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgMproducing cells in their nasal mucosa. Clin ExpImmunol 1987;67:626-36 9 Surjan L Jr, Brandtzaeg P, Berdal P. Tmmunoglobulin systems of human tonsils. 11. Patients with chronic tonsillitis or tonsillar hyperplasia: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol 1978;31:382-90 10 Surgan L Jr. Tonsils and lymphoepithelial structures in the pharynx as immunobarriers. Acta Otoaryngol (Stockh) 1987;103:369-72 11 Brook I, Youson P. Quantitative measurement of B-lactamase in tonsils of children with recurrent tonsillitis. Acta Otolaryngol (Stockh) 1984:536-59 12 Church MK, Norn S, Pao G J-K, Holgate ST. Non-IgEdependent bacteria-induced histamine release from 13 14 15 16 17 18 19 20 human lung and tonsillar mast cells. Clin Allergy 1987;17:341-53 Kaliner M. Hypotheses on the contribution of late-phase allergic response to the understanding and treatment of allergic diseases. J Allergy Clin Immunol 1984; 73:311-15 Paradise JL, Bluestone CD, Bachmann RN, et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and non-randomized clinical trials. N Engl J Med 1984;310:674-83 Mawson SR, Adlington P, Evans M. A controlled study evaluation of adeno-tonsillectomy in children. JLaryng 1967;81:777-90 Sanderson J, Warner JO. Previous ENT surgery in children presenting with allergic perennial rhinitis. Clin Allergy 1987;17:113-17 Donovan R, Soothill JF. Immunological studies in children undergoing tonsillectomy. Clin Exp Immunol 1973;14:347-57 Ogra PL. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody responses to poliovirus. N Engi J Med 1971;284:59-64 Vianna NJ, Greenwald P, Davies JNP. Tonsillectomy and Hodgkin's disease. Lancet 1971;ii:168-9 Langman AW, Kaplan MJ. Hodgkin's disease and tonsillectomy. Otolaryngol Clin North Am 1987;20: 399-404 (Accepted 22 August 1989. Correspondence to Dr Glenis Scadding, 143 Chevening Road, London NW6 6DZ) 107