* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PP076 Allergenicity assessment strategy for novel food proteins and
Survey
Document related concepts
Protein domain wikipedia , lookup
List of types of proteins wikipedia , lookup
Homology modeling wikipedia , lookup
Protein folding wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Western blot wikipedia , lookup
Transcript
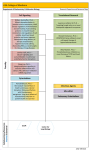
PP076 Allergenicity assessment strategy for novel food proteins and protein sources Kitty Verhoeckx1, Henrike Broekman2, André Knulst2, Geert Houben1 1 The Netherlands Organization for Applied Scientific Research (TNO), Zeist, the Netherlands; 2UMC Utrecht, the Netherlands Aim: Development of an allergenicity assessment strategy for novel proteins and protein sources. Methods: Previously published literature on allergenicity risk assessment, EFSA opinions on novel foods and the use of the “weight-of-evidence approach” for food derived from GM plants were consulted. Results: A new conceptual strategy is developed for assessing the allergenicity of novel proteins (Figure 1). Discussion: Allergenicity risk assessment might pose some major difficulties in case of approval of novel foods on the food market, since detailed guidance on how to assess the allergenic potential of novel foods is not available. At present, the approach relies mostly on the guidance of allergenicity assessment for genetically modified (GM) plant foods. However this guidance is difficult to interpret, not completely applicable or validated for novel foods and therefore needs some adjustments. The allergenicity assessment strategy must address cross reactivity with known allergens and sensitising potency of the novel protein. Conclusion: The proposed strategy gives more guidance on how to assess the allergenicity of novel food proteins and protein sources, as was previously shown for mealworm proteins (Broekman et al). Figure 1: Schematic overview of a new allergenicity assessment strategy of novel proteins and protein containing sources (Verhoeckx et al). References: 1 Verhoeckx K, Broekman H, Knulst A, Houben G, Allergenicity assessment strategy for novel food proteins and protein sources, Regul Toxicol Pharmacol. 2016 Mar 21. E-pub. 2 Broekman H, Verhoeckx KC, den Hartog Jager CF, Kruizinga AG, Pronk-Kleinjan M, Remington BC, Bruijnzeel-Koomen CA, Houben GF, Knulst AC. Majority of shrimp-allergic patients are allergic to mealworm, J Allergy Clin Immunol. 2016 Apr;137(4):1261-3. POSTER SESSION 2: Management of food allergy