* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ATOMS AND THE PERIODIC TABLE chapter three
Survey
Document related concepts
Transcript
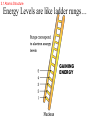
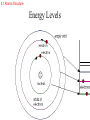
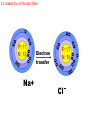
ATOMS AND THE PERIODIC TABLE chapter three 3.1 Atomic Structure ATOMIC THEORY - history 4TH CENTURY B.C. Matter is made of tiny particles called ATOMS. John DALTON EARLY 1900’s (1913) MID 1900’s Modern (after 1925) theory ELEMENTS are made of particles called atoms. ATOMS of the same elements are alike. ATOMS form molecules. ELECTRONS orbit like planets in the solar system. 3.1 Atomic Structure ATOMIC THEORY - STRUCTURE MODEL OF THE ATOM ENERGY LEVELS ELECTRON - NUCLEUS PROTON + NEUTRON (NEUTRAL) 3.1 Atomic Structure ATOMIC STRUCTURE ELECTRON LOCATION Electrons are found in energy levels of an atom. Electrons occupy the lowest energy level available. 3.1 Atomic Structure Energy Levels are like ladder rungs… GAINING ENERGY 3.1 Atomic Structure Energy Levels 3.1 Atomic Structure ORBITALS – where the electrons are located within an energy level. S orbital (Like a sphere) may contain up to 2 electrons first energy level is an s orbital 3.1 Atomic Structure P orbital (Like a dumbbell) may contain up to 2 electrons second energy level may contain an s orbital and up to 3 p orbitals 3.1 Atomic Structure Orbitals 3.1 Atomic Structure Valence electrons are located in the outermost energy level of an atom. They determine the chemical properties of an element. 3.2 A TOUR OF THE PERIODIC TABLE • Properties of elements change in a regular pattern that the table helps to describe. • Periods – Horizontal Rows • Groups(families) – Vertical Columns 3.2 Guided Tour of Periodic Table Traditional Periodic Table 3.2 Guided Tour of Periodic Table The number of protons in an atom determines an element’s location on the table. 3.2 Guided Tour of Periodic Table Elements On the Periodic Table ATOMIC NUMBER 6 PROTONS (and ELECTRONS too) SYMBOL NAME MASS C Carbon 12.001 PROTONS + NEUTRONS MASS # 3.2 Guided Tour of Periodic Table 1 1 PROTONS AND NEUTRONS HAVE EQUAL MASS. ELECTRONS ARE TINY – 1800 EQUAL ONE PROTON. 1800 1 3.2 Guided Tour of Periodic Table MASS ATOMIC NUMBER 35 17 Cl protons _?_ electrons _?_ neutrons _?_ 3.2 Guided Tour of Periodic Table MASS ATOMIC NUMBER 35 17 Cl protons 17 electrons 17 neutrons 35-17 = 18 3.2 Guided Tour of Periodic Table IONS are atoms that have lost or gained an electron. • ELECTRON GAINED = NEGATIVE CHARGE (-) ELECTRON LOST = POSITIVE CHARGE (+) 3.2 Guided Tour of Periodic Table Electron transfer Na+ Cl 3.2 Guided Tour of Periodic Table TWO KINDS OF IONS: A (+) CHARGED ION IS A Cation. A (-) CHARGED ION IS AN Anion. EXAMPLES: • LITHIUM • FLUORIDE 3.2 Guided Tour of Periodic Table The atoms of an element always have the same number of protons. BUT….. The atoms of an element may have different numbers of neutrons. This is an ISOTOPE! Two carbon ISOTOPES: CARBON 12 = 6 protons and 6 neutrons CARBON 14 = 6 protons and 8 neutrons 3.2 Guided Tour of Periodic Table The AVERAGE MASS of an ATOM • Why is the mass number not an even number? – Atoms of the same element exist with different numbers of neutrons. – This makes the mass of different atoms of the same element different. – The average mass is a weighted number so that more common isotopes have a greater affect on the average than rare isotopes. • What is an amu? – It is an “atomic mass unit”. – An amu is equivalent to the mass of 1/12 of a carbon-12 atom. 3.3 FAMILIES OF ELEMENTS: • HAVE THE SAME VALENCE NUMBER. • HAVE SIMILAR CHEMICAL AND PHYSICAL PROPERTIES. • A COLUMN OF ELEMENTS IS A FAMILY. 3.3 Families of Elements TWO MAJOR DIVISIONS • METALS • NONMETALS 3.3 Families of Elements GROUP ONE: ALKALI METALS • VERY REACTIVE • ONE VALENCE ELECTRON 3.3 Families of Elements GROUP TWO: ALKALINE EARTH METALS • TWO VALENCE ELECTRONS 3.3 Families of Elements GROUP 3-12: TRANSITION METALS • MANY COMMON METALS • NOT AS REACTIVE AS OTHER METALS 3.3 Families of Elements GROUP 17: HALOGENS • VERY REACTIVE • FORM SALTS WITH ALKALI METALS • 7 VALANCE ELECTRONS 3.3 Families of Elements GROUP 18: NOBLE GASES • INERT / UNREACTIVE • EIGHT VALENCE ELECTRONS 3.3 Families of Elements SYNTHETIC ELEMENTS • They are man-made and radioactive. • They include all elements above #92, plus #43 and #61. 3.3 Families of Elements SEMICONDUCTORS (METALOIDS) The elements that are between the metals and nonmetals are known as: SEMICONDUCTORS (METALOIDS) They may exhibit metallic and nonmetallic properties. B Si Ge As 3.4 USING MOLES TO COUNT ATOMS • Some counting units: – – – – Reams of paper Dozens of eggs Atomic mass units of protons and neutrons Moles of atoms • A mole is the SI unit that describes the amount of a substance. • Avogadro’s constant is the number of particles in one mole which = 6.022 x1023 • molar mass is the mass in grams of one mol of a substance…it is equal to the average atomic number of an atom. Chapter 3 Studying for the Test • • • • • • Vocabulary Parts of an atom History Element families Using the periodic table Metal vs. nonmetal