* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biology 105
Carbon sink wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosequestration wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
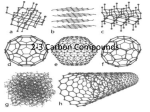
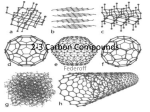
Chapter 3: Chemistry of Life Organic Compounds Pgs 46-73 Student Outcomes Describe the properties of carbon that make it the central component of organic compounds. Explain the relationship between polymers and macromolecules Distinguish among the three types of carbohydrates. Student Outcomes Distinguish among Lipids -fats, phospholipids and steroids. Describe the structure and function of proteins. Describe the components of Nucleic acids. Organic Compounds Covalent bonds of carbon atoms - forms the background of a molecule (carbon-carbon or carbon-hydrogen) Called organic because once thought to only be created by living things. Carbon Atomic Number is 6 - What does this mean? Carbon bonds can form 4 covalent bonds. Ideally suited for bonding to multiple atoms. Very strong bonds Hydrocarbons Carbon-Hydrogen bonds - non polar Insoluble in water - Hydrophobic Types of Organic Molecules Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates Sugars, starches and cellulose Contain Elements: CH20 (ratio) Carbon Hydrogen Oxygen Monosacharides Glucose, Fructose and Galactose Glucose - the most abundant sugar and energy source of most organisms Average American consumes about 120-130 lbs of sugar yearly. Disaccharides Two simple sugars combined Maltose - (glucose + glucose) Sucrose - Table sugar (glucose + fructose) Lactose - Milk Sugar (glucose + galactose) Polysaccharides Long chain of simple sugars – Starch - energy storage in plants, water (hydrolysis) breaks it down into glucose units – Glycogen - animal ‘starch’, more water soluble than plant starch, stored in liver and muscle cells – Cellulose - most abundant carbohydrate, we cannot break it down Modified Carbohydrates Chitin - main component of skeletons of arthropods Glycoproteins -Carbohydrates + proteins (found on outer side of most cells) Glycolipids - Carbohydrates + lipids (found on outer side of animal cells) Artificial Sweetners Aspartame – 200 times sweeter than Sucrose (table sugar) Sucralose (Splenda) – 600 times sweeter than Sucrose Both will pass through the digestive tract unmodified Lugduname is 200,000 times sweeter (sweetest compound known) Lipids Insoluble in water – Fats, phospholipids, carotenoids, steroids, waxes – Elements: Carbon and Hydrogen; Oxygen in a smaller role – Made of fatty acids Saturated fatty acids Have maximum possible number of hydrogen atoms Solid at room temperature - animal fat, butter, shortening Increased risk of cardiovascular disease Hydrogenation Bombarding an oil’s fat molecules with hydrogen atoms, making it more dense, raising its melting point, - solid at room temperature. Known as trans fatty acids. Partially hydrogenated oil means that the hydrogenation process stopped short of a full solid, reaching a more creamy, butterlike consistency = some margarines. (carbon-hydrogen bonds) Unsaturated fatty acids Carbon atoms joined together, so not fully saturated with hydrogen Monounsaturated fatty acids (Oleic acid) Polyunsaturated fatty acids - multiple double carbon bonds Olestra Fat substitute – Tastes and texture is similar to fats, but as with artificial sweeteners, will not break down along the digestive tract. Proteins All proteins in a cell are called its Proteome. Study of Protein structure/activities Proteomics Macromolecules of amino acids – From 9 - 8000 amino acids Enzymes (catalysts) Elements: Carbon, Hydrogen, Oxygen and Nitrogen Amino Acids 20 common amino acids Several are essential - the body cannot synthesize in amounts necessary so must be obtained from diet. Amino acids are joined together - peptide bond Protein structure Different amino acid sequences will dictate the protein’s function. A single mutation can disrupt the entire function. Heat can also ‘denature’ a protein’s shape, making it useless. This change in shape is usually non reversible. Ex: albumin (egg white) Kwashiorkors Enlarged abdomen due to enlarged liver from an accumulation of lipids and not enough protein in the diet to form lipoproteins Common for a diet high in grains and starchy foods and low on protein. Nucleic Acids Transmit hereditary information DNA - Deoxyribonucleic acid or RNA - Ribonucleic acid Elements: Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorous Nucleic Acids Structure Nucleotides that consist of – 5 carbon sugar (deoxyribose/ribose) – Phosphate group – Nitrogenous base (purine /pyrimidine) Nucleotides ATP – Provide energy for all cells – Composed of adenine, ribose and 3 phosphates Graphene-a carbon allotrope Life after silicon Hottest new material in electronics A flat sheet of pure carbon rings, one atom thick that resembles chicken wire. Carbon Carbon’s relationship to greenhouse gasses??? Global Warming 4 questions – 1) Is it really happening? – 2) Are human actions partly responsible? – 3) Will organisms be able to adapt (natural selection) – 4) Will the planet be able to correct itself without human intervention?