* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Antineoplastic Action of Growth Hormone
Neuropharmacology wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Toxicodynamics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
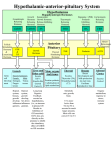
56 Recent Patents on Anti-Cancer Drug Discovery, 2012, 7, 56-63 Antineoplastic Action of Growth Hormone-Releasing Hormone (GHRH) Antagonists Agnieszka Siejka1, Hanna Lawnicka2, Gabriela Melen-Mucha2, Ewelina Motylewska2, Jan Komorowski1 and Henryk Stepien2,* 1 Department of Clinical Endocrinology, Chair of Endocrinology, Medical University of Lodz, 91-425 Lodz, Dr. Sterling str 3, Poland, 2Department of Immunoendocrinology, Chair of Endocrinology, Medical University of Lodz, 91-425 Lodz, Dr. Sterling str 3, Poland Received: December 22, 2010; Accepted: February 9, 2011; Revised: February 28, 2011 Abstract: Some of the antagonists of growth hormone-releasing hormone (GHRH) are able to inhibit the growth of various experimental human cancers. The antitumor effects of first antagonists seemed to be dependent mainly on the disruption of pituitary secretion of growth hormone (GH), followed by the reduction in the levels of circulating insulin-like growth factor (IGF)-1, an important growth factor for cancer cells. It seems obvious, that growth hormone deficiency (GHD) induced by GHRH antagonists with all its complications, could limit the beneficial effects of GHRH antagonists therapy, and decrease patients’ quality of life. The discovery of local autocrine/paracrine production of GHRH and other related growth factors in many tumoral tissues, in combination with the wide expression of GHRH receptors on cancer cells, directed the research to the synthesis of more potent GHRH antagonists. These compounds exert strong inhibitory effects directly on tumor growth, with scarce endocrine action. The receptor-mediated mechanisms comprise complex and still not completely understood effects on intracellular signaling pathways that are strictly related to human tumorigenesis. This review summarizes recent patents and latest observations on the antineoplastic role of GHRH antagonists in human tumors with emphasis on potential therapeutic applications in clinical oncology. Keywords: Cancer therapy, GHRH, GHRH antagonists, IGF-I, IGF-II. INTRODUCTION The existence of growth hormone releasing factor was first suggested by Reichlin in 1961, who demonstrated that bilateral lesions of the ventromedial hypothalamus in rats resulted in impaired linear growth in growing individuals [1]. Despite many efforts, the growth hormone releasing factor was not identified until more than 20 years after. In 1982 two groups of researchers isolated and characterized the 40and 44- amino-acid peptides from pancreatic tumors that induced acromegaly in the absence of a pituitary tumor [2-4]. Discovered later on hypothalamic growth hormone-releasing hormone (GHRH) was eventually shown to be identical with the one which was isolated from the tumors one year before [4-6]. The structure of 44 amino acid peptide was identified as Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile Met-Ser-Arg-Gln-Gln-Gly-Glu-Ser-Asn-Gln-Gly-Arg-Gly Ala-Arg-Ala-Arg-Leu-NH2. *Address correspondence to this author at the Department of Immunoendocrinology, Chair of Endocrinology, Medical University of Lodz, Dr. Sterling 3 Str., 91-425, Lodz, Poland; Tel: +48426324854; Fax: +48426324854; Email: [email protected] -9/12 $100.00+.00 The human GHRH gene has been localized in chromosome 20 [7]. It includes five exons and spans about 10-18 kilobases of genomic DNA. The precursor protein for human GHRH contains 108 amino acids, which can be processed into the 40- or 44-amino acid GHRH peptide [8]. The full biological activity is retained by the N-terminal 29 amino acid fragment [9]. GHRHs isolated from human pancreatic tumor and from hypothalamus were shown to stimulate the secretion of growth hormone from pituitary cells [10-12]. GHRH belongs to the pituitary adenylate cyclaseactivating polypeptide (PACAP)/glucagon superfamily of hormones. This family includes nine hormones: GHRH, glucagon, glucagon-like peptide-1 (GLP-1), GLP-2, glucosedependent insulinotropic polypeptide (GIP), peptide histidine-methionine (PHM), PACAP, secretin, and vasoactive intestinal polypeptide (VIP). These peptides are related by structure, distribution (especially the brain and gut), function (acting often by activation of cAMP), and receptors (a subset of seven-transmembrane receptors) [13]. GHRH is synthesized in central nervous system (CNS), mainly in arcuate and ventromedial nuclei of hypothalamus and then it is released from neuronal projections into the hypothalamic-pituitary portal vascular system [8]. Upon binding to its receptors (GHRHR) it stimulates synthesis and release of growth hormone (GH), and possesses mitogenic activity for pituitary cells [14]. GHRH deficiency can lead to pituitary hypoplasia and dwarfism [15]. Extrahypothalamic distribution of GHRH-containing neurons in the CNS indi© 2012 Bentham Science Publishers GHRH Antagonists in Cancer cates that GHRH may play a role in neuromodulation, such as promoting sleep and food intake [16]. Expression of GHRH has been found in various normal extra-pituitary tissues: ovary, placenta, testis, pancreas, gastrointestinal tract, prostate and immune cells [8, 13, 17]. Since GHRH is secreted only into the hypothalamichypophyseal portal system, the level of this hormone in the plasma is very low. The disappearance half-time for GHRH (1-44)NH2 is only 6 minutes due to degradation by peptidases [8]. The most important role of GHRH is the stimulation of the secretion of GH with resultant increase in the serum concentrations of insulin-like growth factor-1 (IGF-1) and increase in linear growth in growing individuals and many other consequences in not growing ones. Excessive GHRH synthesis results in GH oversecretion and leads to somatotroph hyperplasia and severe metabolic results together with excessive growth (acromegaly or gigantism). Transgenic mice ectopically expressing human GHRH show increased growth during growth phase and pituitary hyperplasia that can often progresses to adenoma in older animals [18]. GHRH receptor (GHRHR) belongs to the family of Gsprotein-coupled receptors that also includes secretin, VIP, glucagon, GLP-1, PACAP, and GIP receptors. GHRHR is a 423 amino acids protein and has seven hydrophobic transmembrane domains. The human GHRH receptor gene has been mapped to chromosome 7. It contains more than 10 exons and spans more than 8 kilobases [19]. The 5-flanking region of GHRHR gene contains several binding sites for transcription factors, such as CREB (cAMP-response element), pituitary-specific transcription factor (Pit-1), nuclear factor-kappa B (NF-B) and other factors such as estrogens (estrogen responsive elements), glucocorticoids (glucocorticoid responsive element) [16, 19]. GHRHR is expressed predominantly in the anterior pituitary gland (pituitary-type GHRHR, pGHRHR) with lower expression in several other tissues, such as placenta and kidney [18]. Very low levels of the mRNA for GHRHR and or protein expression (immunocytochemistry) can be also detected in other tissues, such as gastric mucosa, neuroendocrine cells in colonic mucosa, pancreas, kidney, lung, liver, prostate [20, 21]. Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 57 such as: prostate, breast, ovarian, endometrial, adrenal, lung (both small-cell lung carcinomas [SCLC] and non-SCLC), gastric, colorectal, brain and pancreatic cancers, bone sarcomas, lymphomas and renal carcinomas [17, 26, 27]. The expression of mRNA for GHRH was higher in some of the neoplasms derived from the immune-cells as compared to normal lymphocytes [28]. GHRH has been shown to act as potent mitogen in many of human cancers. It has been also shown to stimulate the proliferation of various cancer cell lines such as prostate, breast, endometrial, ovarian, gastric, pancreatic, colorectal, lung, and osteosarcomas [reviewed in 17, 26, 27, 29]. Knocking down GHRH expression in human prostate, breast and lung cancer cell lines resulted in dramatically decreased cell proliferation, an effect which was abolished after the addition of exogenous GHRH [30]. Therefore, it should be assumed that GHRH can be considered a potent growth factor and that deregulation of GHRH gene transcription might contribute to the pathogenesis of some of human malignancies. Extensive studies report low expression of pituitary-type GHRHRs in only some of human cancers, including lymphomas, glioblastoma and SCLC cell lines and in surgical specimens of human non-small cell lung cancer [17, 21]. The receptors that are commonly expressed in most of the human cancers were identified ten years ago and are splice variants (SVs) of the full length pGHRHR [31, 32]. The major part of the cDNA sequence of SV1 is identical to the corresponding sequence of pGHRHR cDNA, but the first 334 nucleotides of SV1 are different. The protein sequence of SV1 differs from that of pGHRHR only in the N-terminal extracellular domain, where a 25-amino-acid sequence replaces the first 89 amino acids of pGHRHR. The mRNA sequence of SV1 and the observed molecular mass of the receptor protein from western-blot assays are consistent with a seventransmembrane-domain receptor that has the third intracellular loop critical for the interaction with G proteins [17, 31]. Upon binding of GHRH to its receptor, this peptide stimulates the adenylyl cyclase (AC) with resulting increase in intracellular cAMP levels and activation of protein kinase A (PKA). The influx and increased concentration of intracellular calcium causes the release of GH from somatotrophs [8]. There is also direct evidence on the mitogenic action of GHRH and activation of mitogen-activated protein kinase (MAPK) pathway in somatotroph cells [18, 22-24] and in GH3 cells transfected with GHRH receptor [25]. The expression of mRNA for SVs and the protein has been demonstrated in several cancers, including prostate, breast, colorectal, gastric, pancreatic, renal, ovarian and endometrial cancers, adrenal tumors, SCLC and non-SCLC, bone sarcomas, glioblastomas and lymphomas [17, 27]. Furthermore, the induced expression of pGHRHR or SV1 in MCF-7 human breast cancer cells which normally do not produce endogenous GHRH receptors, restores their responsiveness to exogenously given GHRH [33, 34]. Cells transfected with pGHRHR or SV1 responded with increased proliferation rate to GHRH, with SV1 being more potent than GHRHR [34, 35]. Moreover, the cells transfected with SV1 proliferated more quickly even in the absence of exogenous GHRH, suggesting ligand-independent activity of SV1 [3436]. Thus, GHRH receptors can be considered as potential targets for anticancer therapy. GHRH AND RECEPTORS FOR GHRH IN CANCER SYNTHESIS OF GHRH ANTAGONISTS The presence of GHRH and its receptors has been demonstrated in various tumors, suggesting that this neuropeptide may be involved in the pathogenesis of neoplasms as an autocrine/paracrine growth factor [17]. The expression of mRNA for GHRH and/or GHRH protein has been demonstrated in surgical specimens or cell lines of human tumors, Antagonists of growth hormone-releasing hormone (GHRH) have been initially developed for the treatment of disorders caused by excessive synthesis of GHRH or growth hormone (GH). Robberecht et al. demonstrated in 1985 that replacement of Ala at position 2 of GHRH(1-29)NH2 with D-arginine produces GHRH antagonists [37]. This first stan- 58 Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 dard antagonist was able to suppress GH release in rats [37]. It also produced a 30-40% inhibition of GH levels in patients with acromegaly from ectopic GHRH synthesis [38]. The first antagonist (Ac-[Tyr1,D-Arg2]GHRH(129)NH2) was used as a standard for the subsequent development of more potent antagonistic analogs of GHRH which have been developed for the treatment of various cancers [39]. The clinical need for anticancer applications of GHRH antagonists was supported by Pollak et al. on the grounds that somatostatin analogs do not adequately suppress GH and IGF-I levels in patients with neoplasms potentially dependent on IGF-I [40, 41]. Inhibitory effects of these GHRH antagonists have been evaluated in a variety of human experimental cancer models. The first potent antagonists (MZ-series) contained hydrophobic moieties at the N-terminus, the enzyme resistant agmatine at the C-terminus, and other substitutions such as 4-chloro-phenylalanine, 2-aminobutyric acid, and norleucine that increased the receptor-binding affinity and enhanced the stability [42, 43 and reviewed in 17, 39]. Some representatives of this class of antagonists also contained lactam bridge constraints. MZ-4-71 and MZ-5-156 were the most potent antagonists in this series of analogs both in vitro and in vivo. The incorporation of positively charged amino acids into early antagonists led to the JV-series of analogs with further increased bioactivity [39, 44, 45]. Replacement of the native serine by arginine or homoarginine led to development of antagonists with increased activity and binding affinity to the pituitary GHRH receptors. Analog JV-1-36 induced a strong and prolonged inhibition of GH release in vivo for at least 30 min [44]. Antagonistic activity of JV-1-38 for in vivo GH secretion was weak, but still exerted stronger anti-tumor effects as compared to the earlier antagonists. Antagonists JV1-62 and -63 with paraamidinophenylalanine (Amp) at position 10 significantly inhibited GHRH-induced GH release for about 1h in vivo [45]. GHRH antagonist JV-1-65, containing Amp at position 9, did not significantly inhibited GH release in vivo but still decreased the growth of human lung cancer lines xenografted into nude mice more potent than JV-1-63 [46]. This led to the conclusion that antitumor effects of GHRH antagonists are not always related to the endocrine activities on the release of GH from the pituitary [17, 45]. Acylation of JV-peptides by fatty acids at the N-terminus (either monocarboxylic or dicarboxylic, containing six to sixteen carbon atoms) led to development of MZ-J series of antagonists [47]. The binding affinity of these lipopeptide antagonists to GHRH receptors was very high, about 100 fold higher than that of the standard antagonist. These compounds had superior inhibitory effects on the growth of various cancers, including prostate and pancreatic cancers and were demonstrated to cross the blood-brain barrier [48]. Other antagonists containing histidine and ornithine instead of arginine and lysine in positions 11, 12, 20 and 21 of the GHRH peptide (including MZ-J-7-118, MZ-J-7-138 and JMR-132) possessed one of the most potent antitumor effects reported so far [17]. Antagonists with weaker endocrine effects (ex JMR-132) than JV-1-63, MZ-4-71 or MZ-5-156 exhibited greater anticancer potency. This confirmed previous observations that the oncological activity of GHRH an- Siejka et al. tagonists can be based on direct inhibitory effects on cancer cells. In 2009, researchers at Ipsen Pharma S.A.S. disclosed novel series of synthetic antagonistic analogues (WO2009108364). The data revealed that selected peptides could significantly inhibit the GH release from HEK293 cells transfected with GHRHR. Moreover, when administered subcutaneously for 3 weeks, they significantly inhibited the growth of MX-1 (human mammary carcinoma cells), and PC-3 (human prostate cancer cells) xenografted into athymic mice [49]. Data for novel fluorinated synthetic analogs of hGHRH(1-29)NH2 and hGHRH(1-30)NH2 are presented in US20100092539 and US20110066230 [50]. GHRH antagonist MIA-602 (P-12602) studied for 4 weeks (2g/day s.c.) in male nude mice implanted with PC-3 prostate cancer inhibited tumor growth by 65.92% (p<0.001 versus control). When MIA-610 (P-12610) peptide was studied in the same experimental model, it inhibited tumor growth by 77.78% (p<0.001 versus control) [50]. New series of GHRH antagonists have been developed for oncological indications, and have already proven effective in various experimental models (ex PC3 human prostate cancer cells, glioblastoma and breast cancers) [50-52]. Chemical structure of most commonly studied GHRH antagonists is presented in Table 1 and described in detail elsewhere (including US6057422, US7452865, US5550212, US20100092539) [39, 42-45, 47, 50, 53-55]. MECHANISMS OF ACTION OF GHRH ANTAGONISTS The indirect mechanism of inhibition of tumor growth by GHRH antagonists comprises their effect on the GH-IGF-I axis and seems to be an important approach for IGF-Idependent cancers (such as prostate, breast and colorectal cancers). GHRH-antagonists bind to GHRH receptors located on pituitary somatotrophs and block the hypothalamic GHRH-mediated activation of the intracellular cAMP signal transduction pathway. This leads to the inhibition of GH synthesis and release which results in the reduction of IGF-I production from the liver [17, 56]. The first synthesized series of antagonists possessed strong endocrine activity and caused significant decrease in the serum concentrations of IGF-I. However, IGFs (IGF-I and IGF-II) can influence the growth of various human cancers not only through endocrine, but also through autocrine and/or paracrine mechanisms [56, 57]. At least several neoplasms express receptors for IGFs and respective ligands, and proliferate in response to exogenous IGFs in vitro. These include numerous neoplasms, such as prostate, breast, lung and colorectal cancers [57]. In many human cancer lines in vitro and in human cancers xenografted into nude mice, including prostate, renal, pancreatic and colorectal cancers, glioblastomas, ovarian cancers, and non-SCLC, GHRH antagonists inhibited the production of IGF-I and IGF-II and the expression of IGF-II mRNA [17, 39, 56]. However, in H-69 SCLC and other cancers, such as MDA-MB-435 breast cancers, LNCaP prostate cancers, and in HEC-1A endometrial cancers, tumor inhibition observed after GHRH antagonists was not associated with a suppression of IGF-I and IGF-II but due to the blockade of the stimulatory action of autocrine GHRH [17, 58, 59]. In MXT mouse mammary cancer, tumoral GH and GH receptor levels were diminished after treatment with GHRH GHRH Antagonists in Cancer Table 1. Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 59 Structure of Most Commonly Studied GHRH Antagonists [39, 42-45, 47, 50, 53-55]. Standard antagonist [Ac-Tyr1, D-Arg2] hGHRH(1-29)NH2 MZ-4-71 [Ibu-Tyr1, D-Arg2 , Phe(4-Cl) 6, Abu15, Nle27] hGHRH(1-28)Agm MZ-5-156 [PhAc-Tyr1, D-Arg2, Phe(4-Cl)6 , Abu15 , Nle27] hGHRH(1-28)Agm MZ-4-243 [Nac0-Tyr1, D-Arg2, Phe(4-Cl) 6, Abu15, Nle27] hGHRH(1-28)Agm JV-1-10 [PhAc-Tyr1, D-Arg2, Phe(4-Cl)6 , Arg9 , Abu15 , Nle27, D-Arg29] hGHRH(1-29)NH2 JV-1-36 [PhAc-Tyr1, D-Arg2, Phe(4-Cl)6 , Arg9 , Abu15 , Nle27, D-Arg28 , Har29] hGHRH(1-29)NH2 JV-1-38 [PhAc-Tyr1, D-Arg2, Phe(4-Cl)6 , Har9 , Tyr(Me)10, Abu15, Nle27 , D-Arg29] hGHRH(1-29)NH2 MZ-J-7-30 [HOOC-(CH2)12-CO-Tyr1, D-Arg2 , Phe(4-Cl) 6, Arg9, Abu15, Nle27, D-Arg28 , Har29 ] hGHRH(1-29)NH2 MZ-J-7-46 [CH3-(CH2)4-CO-Tyr1, D-Arg2, Phe(4-Cl) 6, Arg 9, Abu15 , Nle 27, D-Arg28, Har29] hGHRH(1-29)NH2 MZ-J-7-110 [HOOC-(CH2)12-CO-Tyr1, D-Arg2 , Phe(4-Cl) 6, Amp9 , Tyr(Me)10, Abu15, Nle27, D-Arg28 , Har29] hGHRH(1-29)NH2 MZ-J-7-114 [CH3-(CH2)6-CO-Tyr1, D-Arg2, Phe(4-Cl) 6, Amp9 , Tyr(Me)10, Abu15 , Nle 27, D-Arg28, Har29] hGHRH(1-29)NH2 JMR-132 [PhAc-Tyr1, D-Arg2, Phe(4-Cl)6 , Ala 8, Har 9, Tyr(Me)10, His11, Abu15, His20 , Nle27, D-Arg28 , Har29] hGHRH(1-29)NH2 MIA-602 [(PhAc-Ada)0-Tyr1, D-Arg2 , Fpa56, Ala8 , Har9 , Tyr(Me)10, His11 , Orn12, Abu 15, His20, Orn 21, Nle27, D-Arg28, Har29] hGHRH(129)NH2 MIA-610 [PhAc0-Tyr1, D-Arg 2, Cpa6 , Ala 8, Har 9, Fpa510, His11 , Orn12, Abu15, His20 , Orn21, Nle27 , D-Arg28, Har 29, Ada30] hGHRH(1-30)NH2 Ibu, Isobutyryl; Nac, 1-Naphthylacetyl; PhAc, Phenylacetyl; Cpa, 4-chloro-Phe; Agm, Agmatine, Decarboxy-Arg; Amp, para-Amidino-Phe; Har, Homo-Arg; Abu, 2-Aminobutyric acid; Tyr(Me), O-Methyl-Tyrosine; Orn, Ornithine; Fpa, Pentafluoro-Phenylalanine; Ada, 12-Aminododecanoyl. antagonists [60]. GHRH antagonists inhibited the proliferation and secretion of vascular endothelial growth factor (VEGF) from mouse endothelial cells cultured in vitro [61]. Subsequently, it was shown that the protein and mRNA levels of tumoral angiogenic growth factors: VEGF and its receptor and/or basic fibroblast growth factor (bFGF), were decreased by GHRH antagonists in human prostate cancer, SCLC and non-SCLC, and lymphoma models [17, 62-64]. In the model of ovarian cancer GHRH antagonist JMR-132 significantly decreased the level of epidermal growth factor receptor (EGFR) and after knocking down of the expression of EGFR by siRNA the antiproliferative effect of JMR-132 was abolished in SKOV3 and CaOV3 cells [65]. Newly synthesized GHRH antagonists MIA-313 and MIA-602 decreased VEGF secretion of OVCAR-3 cells and reduced tumor vascularization in in a Matrigel plug assay [66]. The identification of binding sites for GHRH antagonists confirmed the observations that this group of peptides may also directly inhibit tumor growth. Splice variant 1 of the full length pGHRHR was shown to exhibit strong liganddependent and ligand-independent activity in 3T3 mouse fibroblasts and MCF-7 human breast cancer cells [34, 35]. It has been recently demonstrated that exogenously supplemented GHRH which can act through SV1 receptor strongly activates the MAPK, a pathway associated with the stimulation of cellular proliferation [36]. These observations suggested that the expression of this receptor in tumoral cells may by itself lead to important intracellular changes involving the activation of pro-growth and antiapoptotic pathways. This also supported the efforts for the development of newer antagonists which can target tumor cells directly. The evidence of direct inhibitory effect of GHRH antagonists on the growth of various cancer cell lines, the iden- tification of SVs, and the synthesis of new more potent analogues, initiated studies aiming at the identification of intracellular pathways involved in oncogenesis that are affected by GHRH antagonists. GHRH antagonists can inhibit the PKC-MAPK (protein kinase C-mitogen-activated protein kinase) [53, 67] and PI3K-AKT (phosphatidylinositol 3kinase-protein kinase B) [52] signaling pathways, reduce the expression levels of c-jun and c-fos proto-oncogenes, of wild-type and/or mutant tumor-suppressor protein p53 in human SCLC, non-SCLC, and prostate cancer models [46, 68-71], and decrease the telomerase activity in glioblastomas [72]. GHRH antagonists also reduced the levels of the antiapoptotic Bcl-2 protein and increased levels of BAX (a pro apoptotic protein) and caspases in various models [reviewed in 17]. They also trigger a calcium-dependent apoptotic mechanism in the LNCaP prostate cancer cell line [73]. GHRH antagonists were shown to inhibit proliferation and invasive potential of DBTRG-05 glioblastoma, MDA-MB468 estrogen-independent breast, and ES-2 clear cell ovarian cancers [51]. After treatment with GHRH antagonist (MIA602), the authors observed an inhibition of the release of metalloproteinases (MMP), decreased attachment of cancer cells to fibronectin and matrigel, an upregulation of caveolin-1 and E-cadherin, as well as downregulation of NF-B and beta-catenin [51]. In LNCaP human prostate cancer cell line GHRH antagonist possessed antioxidant activity [74], reducing the levels of lipid and protein oxidative stress markers (MDA and nitrotyrosine) and generation of intracellular reactive oxygen species (ROS) [68]. The data for suppressing the reactive oxygen species (ROS) of certain cells by administration of GHRH antagonists are presented in US20100152114 [74]. GHRH antagonist JMR-132, at the concentration of 1M, influenced the expression of antioxi- 60 Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 dant enzymes, superoxide dismutase (SOD1), which is also a target for the inhibition of angiogenesis and tumor growth, quinine oxidoreductase 1 (NQO1), a cytochrome protein that reduces and detoxifies quinones protecting cells against redox cycling and oxidative stress, GPX1 which is a main glutathione peroxidase and thioredoxin (Trx), a major cytoplasmic antioxidant enzyme [68, 74]. In A549 lung cancer cell line it also decreased the expression of inducible nitric oxide synthase (iNOS) [67], an enzyme involved in the generation of reactive nitrogen species (RNS), and the expression of cyclooxygenase 2 (COX-2) [67, 68, 71]. NOS and COX-2 are enzymes involved in the pathogenesis of inflammatory responses and in oncogenesis as well, linking these two processes, and suggesting also a non-oncological activity of GHRH analogues in various human pathologies [75, 76]. Furthermore, GHRH antagonists were shown to reduce the activity of Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway in experimental model of human prostate hyperplasia, and decreased the proliferation of these cells in vitro [77], as well as to counteract the stimulatory effect of GHRH and the secretion of interleukin17 from human peripheral blood mononuclear cells [78]. Schematic demonstration of selected mechanisms of action of GHRH antagonists is shown in Fig. (1). Fig. (1). Mechanism of Action of GHRH Antagonists. Siejka et al. CURRENT & FUTURE DEVELOPMENTS GHRH antagonists were developed for the controlling the effects of the excessive action of GHRH and GH/IGF-1 that is seen in acromegaly. Further studies with the use of this group of possible future drugs concentrated on their effects on extrapituitary neoplasms. Acromegaly however affects considerable number of the population and in many cases cannot be treated effectively with surgery because of local invasion of the pituitary tumor. Currently, there are three drug classes available for the treatment of acromegaly: dopamine agonists, somatostatin analogs, and a GH receptor antagonist. However, many patients do not respond to this kind of treatment and the responders very rarely may show the desensitisation to the action of somatostatin analogs within weeks or months. Considering various targets for somatostatin analogs and GHRH antagonists, it would be of great interest to evaluate the effects of those peptides applied together in pituitary tumors leading to acromegaly. It is worth emphasizing that GHRH antagonists have been shown to cross the blood-brain barrier [48]. Similarly, patients with neuroendocrine tumors (NETs)/carcinoids whose symptoms are not completely controlled with somatostatin analogs (being currently the main treatment option) might benefit from this type of therapy. The expression of GHRH recep- GHRH Antagonists in Cancer Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 61 tors predominantly in cancer cells allows GHRH antagonists to be used as carrier systems linked to radionuclides for tumor localisation or therapy, or conjugated to chemotherapeutic agents or toxins. So far, only the results of preclinical studies evaluating the anticancer effects of GHRH antagonists are available, but noteworthy this group of synthetic peptides does not cause any significant side effects, especially those of them not having endocrine effects on GH-IGF-I axis. Scarce results in humans with the use of first antagonist did not show any toxicity either. It is intriguiging to speculate that GHRH antagonists might be used as prophylactic agents to decrease the cancer risk, mainly because they can diminish the level of IGFs, but other direct (also metabolic) effects cannot be excluded. IGF = Insulin-like Growth Factor iNOS = Inducible Nitric Oxide Synthase JAK = Janus Kinase MAPK = Mitogen Activated Protein Kinase MDA = Malondialdehyde MMP = Metalloproteinase mRNA = Messenger Ribonucleic Acid NF-B = Nuclear Factor-kappa B Nle = Norleucine Orn = Ornithine In conclusion, though studied only in preclinical models so far, GHRH antagonists create a new potential therapeutic option for various neoplasms. The growing understanding of their mechanisms of action gives new directions to their possible applications. Clinical studies will determine their efficacy in humans. p = Pituitary p-Akt = Akt/phospho-Akt PACAP = Pituitary Adenylate Cyclase-Activating Polypeptide PCR = Polymerase Chain Reaction ABBREVIATIONS PCNA = Proliferating Cell Nuclear Antigen Abu = 2-Aminobutyric acid PhAc = Phenylacetyl Ada = 12-Aminododecanoyl PHM = Peptide Histidine-Methionine Agm = Agmatine, decarboxy-arginine PI3K = Phosphatidylinositol 3-kinases Amp = Para-Amidino-Phenylalanine Pit = Pituitary-Specific Transcription Factor ATP = Adenosine-Tri-Phosphate PKA = Protein Kinase A BAX = Pro-Apoptotic Protein PKC = Protein Kinase C Bcl-2 = Anti-Apoptotic Protein R = Receptor bFGF = Basic Fibroblast Growth Factor ROS = Reactive Oxygen Species c-AMP = Cyclic-Adenosine-Mono-Phosphate SCLC = Small Cell Lung Cancer CREB = cAMP-Response Element siRNA = Silencing Ribonucleic Acid Cit = Citrulline STAT = CNS = Central Nervous System Signal Transducer and Activator of Transcription Cox = Cyclooxygenase SV = Splice Variant Cpa = 4-Chloro-Phenylalanine Tyr(Me) = O-Methyl-Tyrosine DNA = Deoxyribonucleic Acid VEGF = Vascular Endothelial Growth Factor EGFR = Epidermal Growth Factor Receptor VIP = Vasoactive Intestinal Peptide ERK = Extracellular Signal-Regulated Kinase VPAC = VIP and Pituitary Adenylate Cyclaseactivating peptide Fpa5 = Pentafluoro-Phenylalanine G-protein = Guanine nucleotide binding proteins GH = Growth Hormone GHRH = Growth Hormone-Releasing Hormone GIP = Glucose-Dependent Insulinotropic Polypeptide GLP = Glucagon-like peptide h = Human Har = Homoarginine REFERENCES HER = Human EGF Receptor [1] ACKNOWLEDGEMENTS This paper was supported by European Cooperation in the field of Scientific and Technical Research - COST ACTION, Grant No. CM0602 and Polish Ministry of Science and Higher Education, Grant No. 505-04-001. CONFLICT OF INTEREST None. Reichlin S. Growth hormone content of pituitaries from rats with hypothalamic lesions. Endocrinology 1961; 69: 225-30. 62 Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature 1982; 300: 276-8. Guillemin R, Brazeau P, Bohlen P, Esch F, Ling N, Wehrenberg WB. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science 1982; 218: 585-7. Bohlen P, Brazeau P, Bloch B, Ling N, Gaillard R, Guillemin R. Human hypothalamic growth hormone releasing factor (GRF): Evidence for two forms identical to tumor derived GRF-44-NH2 and GRF-40. Biochem Biophys Res Commun 1983; 114: 930-6. Spiess J, Rivier J, Vale W. Characterization of rat hypothalamic growth hormone-releasing factor. Nature 1983; 303: 532-5. Ling N, Esch F, Bohlen P, Brazeau P, Wehrenberg WB, Guillemin R. Isolation, primary structure, and synthesis of human hypothalamic somatocrinin: Growth hormone-releasing factor. Proc Natl Acad Sci USA 1984; 81: 4302-6. Mayo KE, Cerelli GM, Lebo RV, Bruce BD, Rosenfeld MG, Evans RM. Gene encoding human growth hormone-releasing factor precursor: structure, sequence, and chromosomal assignment. Proc Natl Acad Sci USA 1985; 82: 63-7. Frohman LA, Jansson JO. Growth hormone-releasing hormone. Endocr Rev 1986; 7: 223-53. Vance ML. Growth-hormone-releasing hormone. Clin Chem 1990; 36: 415-20. Thorner MO, Rivier J, Spiess J, Borges JL, Vance ML, Bloom SR, et al. Human pancreatic growth-hormone-releasing factor selectively stimulates growth-hormone secretion in man. Lancet 1983; 1: 24-8. Vale W, Vaughan J, Yamamoto G, Spiess J, Rivier J. Effects of synthetic human pancreatic (tumor) GH releasing factor and somatostatin, triiodothyronine and dexamethasone on GH secretion in vitro. Endocrinology 1983; 112: 1553-5. Brazeau P, Ling N, Bohlen P, Esch F, Ying SY, Guillemin R. Growth hormone releasing factor, somatocrinin, releases pituitary growth hormone in vitro. Proc Natl Acad Sci USA 1982; 79: 790913. Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 2000; 21: 619-70. Mayo KE, Hammer RE, Swanson LW, Brinster RL, Rosenfeld MG, Evans RM. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Mol Endocrinol 1988; 2: 606-12. Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature 1993; 364: 20813. Lin-Su K, Wajnrajch MP. Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor. Rev Endocr Metab Disord 2002; 3: 313-23. Schally AV, Varga JL, Engel JB. Antagonists of growth-hormonereleasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab 2008; 4: 33-43. Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res 2000; 55: 237-66; discussion 667. Petersenn S, Rasch AC, Heyens M, Schulte HM. Structure and regulation of the human growth hormone-releasing hormone receptor gene. Mol Endocrinol 1998; 12: 233-47. Hohla F, Moder A, Mayrhauser U, Hauser-Kronberger C, Schally AV, Varga JL, et al. Differential expression of GHRH receptor and its splice variant 1 in human normal and malignant mucosa of the oesophagus and colon. Int J Oncol 2008; 33: 137-43. Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA 2005; 102: 17424-9. Steinmetz R, Zeng P, King DW, Walvoord E, Pescovitz OH. Peptides derived from pro-growth hormone-releasing hormone activate p38 mitogen-activated protein kinase in GH3 pituitary cells. Endocrine 2001; 15: 119-27. Zeitler P, Siriwardana G. Stimulation of mitogen-activated protein kinase pathway in rat somatotrophs by growth hormone-releasing hormone. Endocrine 2000; 12: 257-64. Siejka et al. [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] [42] [43] [44] Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Growth hormonereleasing hormone stimulates mitogen-activated protein kinase. Endocrinology 2000; 141: 2113-9. Lee EJ, Duan WR, Kotlar T, Jameson JL. Restoration of growth hormone-releasing hormone (GHRH) responsiveness in pituitary GH3 cells by adenovirus-directed expression of the human GHRH receptor. Endocrinology 2001; 142: 414-20. Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm 2005; 70: 1-24. Schally AV, Comaru-Schally AM, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, et al. Hypothalamic hormones and cancer. Front Neuroendocrinol 2001; 22: 248-91. Khorram O, Garthwaite M, Golos T. The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J Clin Endocrinol Metab 2001; 86: 3157-61. Kiaris H, Koutsilieris M, Kalofoutis A, Schally AV. Growth hormone-releasing hormone and extra-pituitary tumorigenesis: therapeutic and diagnostic applications of growth hormonereleasing hormone antagonists. Expert Opin Investig Drugs 2003; 12: 1385-94. Barabutis N, Schally AV. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer 2008; 98: 1790-6. Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormonereleasing hormone receptors from human cancers. Proc Natl Acad Sci USA 2000; 97: 10561-6. Halmos G, Schally AV, Varga JL, Plonowski A, Rekasi Z, Czompoly T. Human renal cell carcinoma expresses distinct binding sites for growth hormone-releasing hormone. Proc Natl Acad Sci USA 2000; 97: 10555-60. Siriwardana G, Bradford A, Coy D, Zeitler P. Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol 2006; 20: 2010-9. Barabutis N, Tsellou E, Schally AV, Kouloheri S, Kalofoutis A, Kiaris H. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA 2007; 104: 5575-9. Kiaris H, Chatzistamou I, Schally AV, Halmos G, Varga JL, Koutselini H, et al. Ligand-dependent and -independent effects of splice variant 1 of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA 2003; 100: 9512-7. Barabutis N, Siejka A, Schally AV, Block NL, Cai R, Varga JL. Activation of mitogen-activated protein kinases by a splice variant of GHRH receptor. J Mol Endocrinol 2010; 44: 127-34. Robberecht P, Coy DH, Waelbroeck M, Heiman ML, de Neef P, Camus JC, et al. Structural requirements for the activation of rat anterior pituitary adenylate cyclase by growth hormone-releasing factor (GRF): Discovery of (N-Ac-Tyr1, D-Arg2)-GRF(1-29)-NH2 as a GRF antagonist on membranes. Endocrinology 1985; 117: 1759-64. Jaffe CA, DeMott-Friberg R, Frohman LA, Barkan AL. Suppression of growth hormone (GH) hypersecretion due to ectopic GH-releasing hormone (GHRH) by a selective GHRH antagonist. J Clin Endocrinol Metab 1997; 82: 634-7. Kovacs M, Schally AV, Varga JL, Zarandi M. Endocrine and antineoplastic actions of growth hormone-releasing hormone antagonists. Curr Med Chem 2008; 15: 314-21. Pollak MN, Polychronakos C, Richard M. Insulin-like growth factor I: A potent mitogen for human osteogenic sarcoma. J Natl Cancer Inst 1990; 82: 301-5. Pollak M, Sem AW, Richard M, Tetenes E, Bell R. Inhibition of metastatic behavior of murine osteosarcoma by hypophysectomy. J Natl Cancer Inst 1992; 84: 966-71. Zarandi M, Horvath JE, Halmos G, Pinski J, Nagy A, Groot K, et al. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA 1994; 91: 12298-302. Zarandi M, Kovacs M, Horvath JE, Toth K, Halmos G, Groot K, et al. Synthesis and in vitro evaluation of new potent antagonists of growth hormone-releasing hormone (GH-RH). Peptides 1997; 18: 423-30. Varga JL, Schally AV, Csernus VJ, Zarandi M, Halmos G, Groot K, et al. Synthesis and biological evaluation of antagonists of GHRH Antagonists in Cancer [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] [62] [63] growth hormone-releasing hormone with high and protracted in vivo activities. Proc Natl Acad Sci USA 1999; 96: 692-7. Varga JL, Schally AV, Horvath JE, Kovacs M, Halmos G, Groot K, et al. Increased activity of antagonists of growth hormonereleasing hormone substituted at positions 8, 9, and 10. Proc Natl Acad Sci USA 2004; 101: 1708-13. Kanashiro CA, Schally AV, Groot K, Armatis P, Bernardino AL, Varga JL. Inhibition of mutant p53 expression and growth of DMS153 small cell lung carcinoma by antagonists of growth hormonereleasing hormone and bombesin. Proc Natl Acad Sci USA 2003; 100: 15836-41. Zarandi M, Varga JL, Schally AV, Horvath JE, Toller GL, Kovacs M, et al. Lipopeptide antagonists of growth hormone-releasing hormone with improved antitumor activities. Proc Natl Acad Sci USA 2006; 103: 4610-5. Jaeger LB, Banks WA, Varga JL, Schally AV. Antagonists of growth hormone-releasing hormone cross the blood-brain barrier: a potential applicability to treatment of brain tumors. Proc Natl Acad Sci USA 2005; 102: 12495-500. Dong, Z.X., Deoliveira, D.B. Antagonistic analogues of GHRH. WO2009108364 (2009). Schally, A.V., Varga, J., Zarandi, M., Cai, R.Z., Block, N.L. Fluorinated GHRH antagonists. US2010092539 (2010) & US20110066230 (2011). Bellyei S, Schally AV, Zarandi M, Varga JL, Vidaurre I, Pozsgai E. GHRH antagonists reduce the invasive and metastatic potential of human cancer cell lines in vitro. Cancer Lett 2010; 293: 31-40. Pozsgai E, Schally AV, Zarandi M, Varga JL, Vidaurre I, Bellyei S. The effect of GHRH antagonists on human glioblastomas and their mechanism of action. Int J Cancer 2010; 127: 2313-22. Schally, A.V., Varga, J., Zarandi, M. Antagonistic analogs of GHRH inhibiting IGF-I and -II. US6057422 (2000). Schally, A.V., Varga, J., Zarandi, M., Cai, R.Z. Antagonistic analogs of GH RH. US7452865 (2008). Zarandi, M., Schally, A.V. Analogues of hGH-RH(1-29)NH2 having antagonistic activity. US5550212 (1996). Kineman RD. Antitumorigenic actions of growth hormonereleasing hormone antagonists. Proc Natl Acad Sci USA 2000; 97: 532-4. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008; 8: 915-28. Engel JB, Keller G, Schally AV, Toller GL, Groot K, Havt A, et al. Inhibition of growth of experimental human endometrial cancer by an antagonist of growth hormone-releasing hormone. J Clin Endocrinol Metab 2005; 90: 3614-21. Chatzistamou I, Schally AV, Varga JL, Groot K, Busto R, Armatis P, et al. Inhibition of growth and metastases of MDA-MB-435 human estrogen-independent breast cancers by an antagonist of growth hormone-releasing hormone. Anticancer Drugs 2001; 12: 761-8. Szepeshazi K, Schally AV, Armatis P, Groot K, Hebert F, Feil A, et al. Antagonists of GHRH decrease production of GH and IGF-I in MXT mouse mammary cancers and inhibit tumor growth. Endocrinology 2001; 142: 4371-8. Siejka A, Lawnicka H, Komorowski J, Schally AV, Stepien T, Krupinski R, et al. GH-RH antagonist (MZ-4-71) inhibits VEGF secretion and proliferation of murine endothelial cells. Life Sci 2003; 72: 2473-9. Heinrich E, Schally AV, Buchholz S, Rick FG, Halmos G, Mile M, et al. Dose-dependent growth inhibition in vivo of PC-3 prostate cancer with a reduction in tumoral growth factors after therapy with GHRH antagonist MZ-J-7-138. Prostate 2008; 68: 1763-72. Kanashiro CA, Schally AV, Zarandi M, Hammann BD, Varga JL. Alterations of EGFR/HER, angiogenesis and apoptosis pathways Recent Patents on Anti-Cancer Drug Discovery, 2012, Vol. 7, No. 1 63 [64] [65] [66] [67] [68] [69] [70] [71] [72] [73] [74] [75] [76] [77] [78] after therapy with antagonists of growth hormone releasing hormone and bombesin in non-small cell lung cancer. Int J Oncol 2007; 30: 1019-28. Keller G, Schally AV, Groot K, Toller GL, Havt A, Koster F, et al. Effective treatment of experimental human non-Hodgkin's lymphomas with antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA 2005; 102: 10628-33. Guo J, Schally AV, Zarandi M, Varga J, Leung PC. Antiproliferative effect of growth hormone-releasing hormone (GHRH) antagonist on ovarian cancer cells through the EGFR-Akt pathway. Reprod Biol Endocrinol 2010; 8: 54. Klukovits A, Schally AV, Szalontay L, Vidaurre I, Papadia A, Zarandi M, Varga JL, et al. Novel antagonists of growth hormonereleasing hormone inhibit growth and vascularization of human experimental ovarian cancers. Cancer 2011; Jul 12 DOI: 10.1002/cncr.26291 [Epub ahead of print] Barabutis N, Siejka A, Schally AV. Growth hormone releasing hormone induces the expression of nitric oxide synthase. J Cell Mol Med 2011; 15: 1148-55. Barabutis N, Schally AV. Antioxidant activity of growth hormonereleasing hormone antagonists in LNCaP human prostate cancer line. Proc Natl Acad Sci USA 2008; 105: 20470-5. Stangelberger A, Schally AV, Varga JL, Zarandi M, Cai RZ, Baker B, et al. Inhibition of human androgen-independent PC-3 and DU145 prostate cancers by antagonists of bombesin and growth hormone releasing hormone is linked to PKC, MAPK and c-jun intracellular signalling. Eur J Cancer 2005; 41: 2735-44. Kanashiro CA, Schally AV, Zarandi M, Hammann BD, Varga JL. Suppression of growth of H-69 small cell lung carcinoma by antagonists of growth hormone releasing hormone and bombesin is associated with an inhibition of protein kinase C signaling. Int J Cancer 2004; 112: 570-6. Hohla F, Schally AV, Kanashiro CA, Buchholz S, Baker B, Kannadka C, et al. Growth inhibition of non-small-cell lung carcinoma by BN/GRP antagonist is linked with suppression of KRas, COX-2, and pAkt. Proc Natl Acad Sci USA 2007; 104: 18671-6. Kiaris H, Schally AV. Decrease in telomerase activity in U-87MG human glioblastomas after treatment with an antagonist of growth hormone-releasing hormone. Proc Natl Acad Sci USA 1999; 96: 226-31. Rekasi Z, Czompoly T, Schally AV, Boldizsar F, Varga JL, Zarandi M, et al. Antagonist of growth hormone-releasing hormone induces apoptosis in LNCaP human prostate cancer cells through a Ca2+-dependent pathway. Proc Natl Acad Sci USA 2005; 102: 3435-40. Schally, A.V., Barabutis, N. Antioxidant activity of GH-RH antagonists. US20100152114 (2010). Barabutis N, Schally AV. Growth hormone-releasing hormone: extrapituitary effects in physiology and pathology. Cell Cycle 2010; 9: 4110-6. Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab 2011; 22: 311-7. Siejka A, Schally AV, Block NL, Barabutis N. Antagonists of growth hormone-releasing hormone inhibit the proliferation of human benign prostatic hyperplasia cells. Prostate 2010; 70: 108793. Stepien T, Lawnicka H, Komorowski J, Stepien H, Siejka A. Growth hormone-releasing hormone stimulates the secretion of interleukin 17 from human peripheral blood mononuclear cells in vitro. Neuro Endocrinol Lett 2010; 31: 852-6.