* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download c - Purdue Physics
Coherence (physics) wikipedia , lookup
Speed of gravity wikipedia , lookup
Electromagnetic mass wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Gravitational wave wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Time in physics wikipedia , lookup
Diffraction wikipedia , lookup
Photon polarization wikipedia , lookup
First observation of gravitational waves wikipedia , lookup
Effects of nuclear explosions wikipedia , lookup
Electromagnetism wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
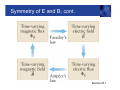
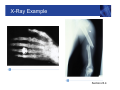
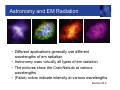
Symmetry of E and B, cont. Section 23.1 Electromagnetic Waves • Self-sustaining oscillations involving E and B are possible • The oscillations are an electromagnetic wave • Electromagnetic waves are also referred to as electromagnetic radiation • Both the electric and magnetic fields must be changing with time • Although Maxwell worked out the details of em waves in great mathematical detail, experimental proof of the existence of the waves wasn’t carried out until 1887 Section 23.1 EM Waves are Transverse Waves • Imagine a snapshot of the electromagnetic wave • The electric field is along the x-axis • The wave travels in the z-direction • Æ E x B, vector cross product • Determined by the right-hand rule #2 • The magnetic field is along the y-direction • Because both fields are perpendicular to the direction of propagation, the wave is a transverse wave Section 23.2 Light is an EM Wave • Maxwell found the speed of an EM wave can be expressed in terms of two universal constants • Permittivity of free space, εo • Magnetic permeability of free space, μo • The speed of an EM wave is called c c= 1 ε o μo • Inserting the values, c = 3.00 x 108 m/s • The value of the speed of an electromagnetic wave is the same as the speed of light Section 23.2 EM Waves in Material Substances • When an em wave travels through a material substance, its speed depends on the properties of the substance. Density of electrons, which interact with the wave and slow it down • The speed of the wave, in “non-vacuum” is always less than c • The speed of the wave depends on the wave’s frequency – because the atomic electrons elastically bound in atoms have resonant frequencies. For frequencies near resonance, the EM wave is slowed down even more. • Dispersion – waves of different freq. get out of step Section 23.2 EM Waves Carry Energy • An em wave carries energy in the electric and magnetic fields associated with the wave Section 23.3 EM Waves Carry Energy, cont. • The total energy per unit volume in the EM wave is the sum of its electric and magnetic energies • utotal = uelec + umag uelec 1 1 2 2 = ε o E and umag = B 2 2 μo Section 23.3 EM Waves Carry Energy, final • The electric and magnetic energies are equal and this leads to the proportionality between the peak electric and magnetic fields 1 1 2 2 ε o Eo = Bo 2 2 μo Eo = c Bo • Remember F = qE = q v X B so it’s pretty natural to find that E = cB since c is a velocity, (and a very special velocity.) Section 23.3 Intensity of an EM Wave • The strength of an em wave is usually measured in terms of its (energy) intensity • SI unit is W/m2 • Intensity is the amount of energy transported per second across a surface of area one square meter. • Intensity also equals the energy density (per unit volume) multiplied by the speed of the wave • I = utotal × c = ½ εo c Eo2 • Since E = c B, the intensity is, equivalently, proportional to the square of the magnetic field amplitude Section 23.3 Solar Cells • The intensity of sunlight on a typical sunny day is • • • • about 1000 W/m² A solar cell converts the energy from sunlight into electrical energy Current photovoltaic cells capture only about 15% of the energy that strikes them Also must account for nights and cloudy days Making better and more practical solar cells is an important engineering challenge. Purdue is a center of intense research on this, with a large interdisciplinary team involved. Section 23.3 EM Waves Carry Momentum • An electromagnetic wave has no mass, but it does carry momentum p • Photons have energy E E = pc • Consider the collision of photons (wave) with matter – total absorption in this case • The momentum is carried by the wave before the collision and by the matter after the collision Section 23.3 Radiation Pressure • When an electromagnetic wave is absorbed by an • • • • object, it exerts a force on the object, since it is transferring momentum to the object F = rate of change of p with time: “N2” The total force on the object is proportional to its exposed area: Energy/time = Intensity x Area Radiation pressure is the radiation Force / Area This can also be expressed in terms of the intensity Pradiation F I = = A c Section 23.3 Electromagnetic Spectrum • All em waves travel through a vacuum at the speed c • c = 2.99792458 x 108 m/s ~ 3.00 x 108 m/s • c is defined to have this value and the value of a meter is derived from this speed –> 1m = distance travelled in a certain fraction of a second. • Electromagnetic waves are classified according to their frequency and wavelength • The wave equation applies to EM waves: c = ƒ λ • The range of all possible electromagnetic waves is called the electromagnetic spectrum Section 23.4 EM Spectrum, Diagram Visible is ~ one octave = factor of 2 in f Section 23.4 EM Spectrum, Notes • There is no defined lower or upper limit for electromagnetic wave frequencies • The range of frequencies assigned to the different types of waves is somewhat arbitrary • Regions may overlap • The names of the different regions were chosen based on how the radiation in each frequency interacts with matter and on how it is generated Section 23.4 Radio Waves • Frequencies from a few hertz up to about 109 hertz • Corresponding wavelengths are from about 108 meters to a few centimeters • Usually produced by an AC circuit attached to an antenna • A simple wire can function as an antenna • Antennas containing multiple conducting elements or shaped as “dishes” are usually more efficient and more common • Radio waves can be detected by an antenna similar to the one used for emitting them Radio Waves, cont. • Parallel wires can act as an • • • • antenna The AC current in the antenna is produced by time-varying electric fields in the antenna This then produces a timevarying magnetic field and the EM wave As the current oscillates with time, the charges are accelerated In general, when an electric charge is accelerated, it produces electromagnetic radiation Section 23.4 Microwaves • Microwaves have frequencies between about 109 Hz and 1012 Hz • Corresponding wavelengths are from a few cm to a few tenths of a mm • Microwave ovens generate radiation with a frequency near 2.5 x 109 Hz • The microwave energy is transferred to water molecules in the food, heating the food Section 23.4 Infrared • Infrared radiation has • • • • frequencies from about 1012 Hz to 4 x 1014 Hz Wavelengths from a few tenths of a mm to a few microns We sense this radiation as heat Blackbody radiation from objects near room temperature falls into this range Also useful for monitoring the Earth’s atmosphere Section 23.4 Visible Light • Frequencies from about 4 x1014 Hz to 8 x1014 Hz • Wavelengths from about 750 nm to 400 nm • The color of the light varies with the frequency • Low frequency; high wavelength – red • High frequency; low wavelength – blue • The speed of light inside a medium depends on the frequency of the radiation • The effect is called dispersion • White light is separated into different colors Section 23.4 Dispersion Example Section 23.4 Ultraviolet • Ultraviolet (UV) light has frequencies from about 8 x 1014 Hz to 1017 Hz • Corresponding wavelengths are about 400 nm to 3 nm • UV radiation stimulates the production of vitamin D in the body, also tanning • Excessive exposures to UV light can cause sunburn, skin cancer and cataracts Section 23.4 X-Rays • Frequencies from about 1017 Hz to about 1020 Hz • Discovered by Wilhelm Röntgen in 1895 • X-rays are weakly absorbed by skin and other soft tissue and strongly absorbed by dense material such as bone, teeth, and metal • X ray images of interior of body • In the 1970’s CT (CAT) scans were developed – 3-D imaging, but at the “cost” of unpleasantly high radiation dosages to the patient – greater than a year’s exposure to cosmic rays, radon, and other natural radiation Section 23.4 X-Ray Example Section 23.4 CT Scan • With a single X-ray image, there will always be parts of the person’s body that are obscured • Images can be taken from different angles • A CT scan takes many Xray images at many different angles • Computer analysis is used to combine the images into a three-dimensional representation of the object Section 23.4 Gamma Rays • Gamma rays are the highest frequency • • • • electromagnetic waves, with frequencies above 1020 Hz Wavelengths are less than 10-12 m Gamma rays are produced by processes inside atomic nuclei – changes in nuclear energy levels One type of radioactive element decay. Including decays of fission products produced in nuclear power plants. Gamma rays also reach us from outside the solar system – one class of “Cosmic Rays”” Section 23.4 Astronomy and EM Radiation • Different applications generally use different wavelengths of em radiation • Astronomy uses virtually all types of em radiation • The pictures show the Crab Nebula at various wavelengths • (False) colors indicate intensity at various wavelengths Section 23.4