* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Problem Set 3_Chem165_Sp2014
History of molecular theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Isotopic labeling wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Process chemistry wikipedia , lookup
Aromatization wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Biochemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
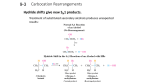
Chem 165 Spring14 Chem 165 Homework #3 due in class Monday April 21 Problems – are from the posted McMurray Chapters 3 and 4 (the latter in two parts) 1. McMurray Problem 3.11 (a) answer for the polarity of the C=O bond; (b) the C–O bonds; (d). 2. McMurray Problem 3.12. 3. McMurray Problem 3.15. 4. McMurray Problem 3.16 is based on the Arrhenius equation k = Ae–Ea/RT, which is discussed in Oxtoby in Section 18.5. Based on that discussion, explain why this question is incomplete (or flawed, depending on your perspective). Give the answer McMurray wants and explain why it is incomplete. 5. McMurray Problem 4.3. 6. McMurray Problem 4.5. Give all three possible answers for (a) and both answers for (b) and (c). For (c), which isomer of the alcohol product do you think you get from the hydration reaction, or do you think you get both isomers? 7. Predict the products of the following reactions. (No reaction could be the correct answer.) (a) 1-octene + MeOH (with an acid catalyst) (b) styrene + excess H2 (over a metal catalyst) (c) cyclopentene + sodium methoxide (an ionic compound containing Na+ and CH3O–) (d) cyclopentene + Br2. Which isomer is made? Is it chiral? (e) the product from part (d) plus one equivalent of sodium methoxide (assume an SN2 mechanism) 8. To make the rac (racemic) isomer, would you add Br2 to the cis or the trans isomer of 4-octene? 9. For carbocations composed of just carbon and hydrogen, the stability is tertiary > secondary > primary > methyl (R3C+ > R2CH+ > RCH2+ > CH3+), as discussed in class. This is the origin of the Markovnikov selectivity observed for electrophilic additions to alkenes. The posted McMurray Chapter 3 is a very good introduction to carbocation reactions, and to organic reactions in general. Markovnikov selectivity is discussed in the following Chapter 4 part 1. Other substituents can effect carbocation stability as well. It is very difficult to form carbocations at carbons bound to electron withdrawing substituents such as a nitro group (–NO2), chlorine atoms, or a carbonyl group [–C(=O)R]. These substituents are “electron withdrawing” because of the electronegativity of the atoms. Yet alkoxy (RO–) and amino (R2N–) substituents strongly stabilize carbocations. In this question you’ll work out why that is. (a) Draw the carbocation formed by addition of H+ to ethyl vinyl ether. (Vinyl is the group derived from ethylene, H2C=CH–.) What is the chemical formula (CxHy…) of this carbocation? (b) Indicate any lone pairs in your carbocation drawing. If there is a lone pair next to the electron deficient (6-electron) carbon, they could make a bond. Draw this second resonance structure, in which the positive charge is formally on the oxygen, not on the carbon. Show by “arrow pushing” how this other resonance form is made. This resonance structure – the partial bond – stabilizes the carbocation. (c) Is this bond a σ bond or a π bond? Do you think that the stabilizing effect of alkoxy and amino groups is based on the polarity of their σ bonds to carbon, or to a π-bonding effect? (d) Based on these arguments, which stabilizes a carbocation better, an amino group or an alkoxy group? [Hint: which is a better base (more readily shares its electron pair)?] (e) Since a second resonance structure stabilizes a carbocation, in part by delocalizing (spreading out) the positive charge, do you think a vinyl group stabilizes a carbocation or destabilizes it? In other words, is the 3-propenyl carbocation (called the allyl cation) more or less stable than the 1-propyl carbocation? This question is answered in the posted McMurray chapter 4 part 2 Sections 4.10, 4.11. Chem 165 Spring14 10. (a) Propose a synthesis to convert cyclohexene into 1,2-dimethoxycyclohexane using any one-carbon compound (CH3X, CH3OH, …) and any non-carbon reagents you want. (b) Do you think your synthesis will give the cis or the trans isomer of the product, or both? 11. Propose a synthesis to prepare ethyl acetate only from ethylene and non-carbon reagents. 12. Propose a synthesis to prepare 2-butyl acetate only from ethylene and non-carbon reagents. 13. Which is easier to make from propene, acetone or propionic acid? Propose a synthesis for the easier one and explain why it is easier. 14. Predict the product of the acid-catalyzed hydration of the alkene below. Note that –NO2 is an electron withdrawing substituent, and –OMe is an electron donating substituent. Explain your reasoning. Does the product have a chiral center? (If there is a chiral center, the product would be a racemic mixture because you are starting from achiral starting materials. In general, to make a product with an excess of one enantiomer over the other, one would have to use some sort of an enantiomericaly enriched starting material, reagent, or catalyst.) OMe O2N 15. In class we passed around some relatively simple chemical compounds for you to smell. Read the two news from Chemical and Engineering News stories on food chemistry posted with this problem set (“Food Detectives” and “The Maillard Reaction Turns 100”). (a) The Food Detectives story refers to a technique called gas chromatography in which a mixture of compounds is volatilized (evaporated into the gas phase) into an inert gas stream that is flowing down a long thin tube packed with material. The different compounds in the mixture move at different rates down the tube, so they are separated by the time they reach the end of the tube (see: http://en.wikipedia.org/wiki/Gas_chromatography). Coupling this to a mass spectrometer gives (in many cases) the molecular weight of each of the components of the mixture. The story also discusses Nuclear Magnetic Resonance (NMR) spectroscopy, which is described in Oxtoby section 20.4. There is a lot of theory in this section on how this works, explaining that this technique looks at the energy level difference between “spin up” and “spin down” nuclei. For our purposes here, we can just take the analysis as pattern recognition, that certain kind of compound will give a resonance in the NMR spectrum at a certain chemical shift. The “Sweet Signs” box in this news story shows a plot of proton (1H) vs. carbon (13C) chemical shift. The vertical axis of this this figure is incorrectly labeled as 12C. What are the nuclear spins of the proton (1H) and the 12C and 13C isotopes? Can you explain why 12C doesn’t give an NMR spectrum? Pick one of the compounds in the “Sweet Signs” figure and give its line structure. All of these are carbohydrates (sugars), as you can tell by the “–ose” suffix. Carbohydrates are discussed in Oxtoby in Section 23.4 (pp. 1120-1122). (b) The article on the Maillard reaction mentions an influential paper from scientists in Sweden, DOI: 10.1021/jf020302f. Download this article and have a look at it. (i) How does this peer-reviewed, scientific paper differ from the news story about the Maillard reaction? (ii) What is the message of this paper? Do you believe it? (iii) Can you tell from Google Scholar or from the ACS publications website how many times this paper has been cited by other scientific papers? The average paper is cited only about 2-3 times. Chem 165 Spring14 Is this an average paper, or has it had a big impact (meaning lots of people have read it and thought it important enough to cite in their papers)? 16. Look at the very recent article in the [very prestigious] journal Science “Structural Insights into Ubiquinone Biosynthesis in Membranes” W. Cheng, W. Li Science 2014, 343, 878-881. (a) Where was this work done? Do you think these authors teach undergraduate classes like Chem 165? (b) In this question we’ll look mostly at the organic chemistry; maybe in the next problem set we’ll look at the biochemistry. The chemistry is nicely summarized in Figure 1A (the top of Figure 1). Does cleavage of the P–O bond in the compound labeled IPP seem reasonable to you? Does it make a relatively stable carbocation? Question 9 above will be helpful to you here. (c) The net chemistry is the substitution of a hydrogen on the benzene ring with the prenyl cation (the chain extending to the right under “Carbocation” in the middle of Figure 1A. This is called an electrophilic aromatic substitution. Explain why this is electrophilic which is opposite to the nucleophilic (SN1/SN2) substitution reactions described in class. (d) The name of the arene substrate, para-hydroxybenzoate (PHB), indicates that the hydroxyl group is opposite (para) to the carboxylate (~CO2–) group of the benzoate. Actually, what is drawn in Figure 1A is the benzoic acid; the benzoate is the deprotonated form. See http://en.wikipedia.org/wiki/Carboxylic_acid. The carbocation attacks the 3-position of the PBH. Assuming that the carbocation attacks a carbon that is bound to a hydrogen, how many other possible places could it attack? Note that some of the positions may be equivalent (recall that there are two resonance structures for the π bonding in the benzene ring). (e) Explain why the carbocation attacks at the 3 position. Draw this carbocation in two possible resonance forms, with the + charge in two different places. Does either one of these place the carbocation on a carbon that has a group that could stabilize it? [See question 9 above.] Give an argument analogous to the Markovnikov selectivity for addition to alkenes. (f) The chemical explanation you gave in part (e) is, however, only part of the story. This reaction happens within the catalytically active site of an enzyme. The structure of that enzyme with PHB and an “uncleavable” analog of IPP, called GSPP, is shown in Figure 2A. The uncleavable analog is used so that the enzyme will stop at this point in the mechanism and let the authors solve the structure (using X-ray diffraction). (i) If you wanted to make an analog of IPP that wouldn’t cleave its P–O bond, what chemical structure would you choose? (ii) In Figure 2A, what is the yellow atom next to C1 in the GSPP? In the mechanism, what is the role of C1? Is it in the right place? Is the PHB in the structure in the same orientation as the PHB drawn in Figure 1A? (iii) Is it possible that the selectivity of the electrophilic substitution is not controlled by the intrinsic organic chemistry, discussed in part (e) above, but rather that it is controlled by the orientation of the substrates within the enzyme pocket?