* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Carbocation Rearrangements
2-Norbornyl cation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
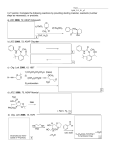
9-3 Carbocation Rearrangements Hydride shifts give new SN1 products. Treatment of substituted secondary alcohols produces unexpected results: Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. Hydride shifts are very fast (faster than SN1 or E1) which is partially due to hyperconjugation in the carbocation weakening the C-H bond): Primary carbocations are too unstable to be formed by rearrangement. Secondary or tertiary carbocations equilibrate readily, leading to a mixture of products when trapped by a nucleophile. Carbocation rearrangement takes place regardless of the precursor leading to the carbocation. Carbocation rearrangements also give new E1 products. Under conditions favoring elimination (elevated temperatures and nonnucleophilic media), carbocations can also rearrange to give rearranged products. Other carbocation rearrangements are due to alkyl shifts. Alkyl shifts, rather than hydride shifts, can occur when a carbocation lacks a suitable secondary or tertiary hydrogen next to the positively charged carbon. Alkyl and hydride shifts are faster when leading to a tertiary carbocation than when leading to a secondary carbocation. In the previous haloalkane rearrangement, movement of an ethyl group rather than a hydride would result in a secondary rather than a tertiary carbocation. Exceptions to this general rule can occur as a result of electronic stabilization or steric relief. Primary alcohols may undergo rearrangement. Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. In this case, steric hindrance interferes with direct attack by the bromide ion. Instead, water leaves at the same time as the methyl group migrates, bypassing the formation of a primary carbocation.