* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download SN1 vs. SN2 Reactions - Master Organic Chemistry
Survey
Document related concepts
Marcus theory wikipedia , lookup
Stille reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
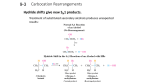
SN1 Reaction SN2 Reaction Stereochemistry Substitution occurs with a mixture of retention and inversion at a stereocenter Br 3 1 2 HO H 2O 3 1 2 retention 2 1 4 2 3 4 2 3 Rate = k [R–Br] + H 3O Br 1 1 2 3 [ 1 4 Br ] [ 2 3 (doubling the concentration of water has no effect on the rate) 4 H 2O ] Fastest for tertiary, slowest for primary Tertiary 2 3 50°C 1 Br Secondary 2 3 1 3 1 50°C 1 3 4 –H Step 3: deprotonation H HO 2 1 3 4 Path A OH 2 1 H Stepwise - leaving group leaves (slow) forming a carbocation, which is then attacked by a nucleophile (fast) H 2O 2 (R) 1.2 × 10 6 11.6 + H 3O Br 1 1 + H 3O Br 2 (likely occuring through SN 2 mechanism) From "March's Advanced Organic Chemistry", 5th Ed. p. 431 Mechanism H 1 OH H 2O 2 2 Rate + H 3O Br OH 50°C 1 2 3 H 2O Br Primary HO H 2O H 2 Br 3 1 Bonds Broken C2 –Br C2 –CN One stereoisomer The rate of the reaction is sensitive to the concentration of the substrate AND the nucleophile Br 4 2 3 CN C N Na 4 1 3 2 Rate = k [R–Br] [ + NaBr 1 1 OH 2 Path B 3 OH 2 2 4 (S) –H Step 3: deprotonation 1 3 4 H 2 OH (S) Path B gives retention (S) "Big Barrier" 1 2 [ 3 4 Br 1 2 3 (doubling the concentration of CN doubles the rate) 4 [ :CN ] ] Unimolecular Bimolecular (substrate only) (substrate and nucleophile) Carbocation stability 3° > 2° >>1° (fastest) Steric hindrance 1° > 2° >>3° (fastest) Weak (generally neutral) Strong (generally bearing a negative charge) Solvent Polar protic (e.g. alcohols) Polar aprotic (e.g. DMSO, acetone) Stereochemistry Mix of retention and inversion Inversion Comparing SN1 vs. SN2 reactions Slowest for tertiary, fastest for primary (methyl even faster) Br Tertiary 4 Na 2 3 4 4 3 2 3 Methyl 2 1 H 3C NC C N 4 1 Br Secondary Primary SN2 Nucleophile Rate Na 4 1 Br Br Na Na 3 2 1 C N 4 2 3 2 3 C N Rate < 0.001 CN C N 1 1 1 H 3 2 4 (S) Br The key skill to start with is identifying the leaving group Look for halogens (Cl, Br, I) or tosylates/mesylates (OTs, OMs) Alternatively, look for alcohols (OH) if acid is present Once you've identified the leaving group, instpect the carbon it is attached to. How many carbons is that carbon connected to? That will tell you if the carbon is primary, secondary, or tertiary. If there are no attached carbons, that's the special case of "methyl" (SN 2 for sure!) CN ~20 If the carbon is tertiary, it's likely SN1. You can rule out SN 2 due to steric hindrance. If the carbon is primary, it's likely SN 2. You can rule out SN1 due to the fact that primary carbocations are unstable [one exception: resonance stabilized carbocations]. CN ~1000 Next, examine the nucleophile. A negatively charged nucleophile generally indicates an SN 2 reaction. A neutral nucleophile (such as H 2O or ROH) generally indicates an SN1 reaction. 1 H 3C 1 N C H Rate Law CN] Finally, check the solvent. A polar aprotic solvent (such as DMSO, acetone, acetonitrile, or DMF) generally indicates SN 2, whereas a polar protic solvent such as H 2O or ROH generally indicates SN1 conditions. In the "backside attack", the nucleophile attacks the substrate from the backside in a single step, resulting in inversion of configuration. Br SN1 Alkyl halide (electrophile) Rate Law One step (backside attack) (S) alkyl halide Carbocation 4 (R) Path A gives inversion (R) Br + Na Mechanism Step 1: Loss of leaving group (slow) Step 2: Attack of nucleophile on carbocation (fast) Can occur from either side of the flat carbocation (Path A or Path B) 3 2 Substrate Substrate Br 4 1 Rate Rate Rate 3 Bonds Formed CN C N Na One stereoisomer inversion HO 1 4 2 inversion! This substitution reaction results in an inversion of configuration at C-2 The rate of the reaction is ONLY sensitive to the concentration of the substrate (and not the nucleophile) H 2O Br + H 3O Br Rate Law Br Stereochemistry Substitution occurs with inversion of configuration at chiral centers OH 3 SN1 vs. SN2 Summary δ N C – H δ + δ– Br partial bonds! Transition state H N C 2 1 3 4 (R) If you found this useful, click here to check out more great organic chemistry “cheat sheets” ! • Explains bimolecular rate law (depends on conc. of nucleophile and substrate) • Explains inversion of stereochemistry • Explains sensitivity to steric hindrance (bulky groups slow down backside attack) This is called the SN2 mechanism (Substitution, Nucleophilic, bimolecular) This sheet Copyright 2015 MasterOrganicChemistry.com Questions, comments? [email protected]