* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Interactions of KCNE Auxiliary Subunits with K and other Channels
Cell membrane wikipedia , lookup
Node of Ranvier wikipedia , lookup
Endomembrane system wikipedia , lookup
Action potential wikipedia , lookup
Signal transduction wikipedia , lookup
List of types of proteins wikipedia , lookup
Membrane potential wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
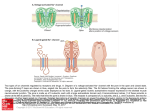
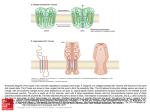
Interactions of KCNE Auxiliary Subunits with Kv and other Channels Alon Meir, Ph.D. KCNEs are integral membrane proteins that associate with and strongly modulate the activity of several ion channels. The different KCNE isoforms are widely and differentially expressed in muscular and neuronal tissues as well as in epithelial cells. Mutations in KCNE genes were shown to lead to disruptions of diverse physiological systems and diseases such as cardiac arrhythmias, deafness and paralysis. It is becoming increasingly apparent that KCNE auxiliary subunits are capable of interacting with many different ion channels. These interactions are the scope of this brief review. Functional voltage dependent K+ channels are constructed primarily by tetramerization of Kv α subunit pore forming proteins (Figure 1). With about 40 genes encoding Kv channel subunits, which may ensemble by either homo or heterotetramerization, the molecular and functional diversity of native voltage dependent K+ currents is huge.1 In addition, several groups of proteins referred to as auxiliary subunits were shown to interact and modify Kv currents. These include Kvβ, KChiP and KCNE proteins. As each of these consists of several isoforms and each pore forming tetramer may interact with different combinations of auxiliary subunits, further Kv current heterogeneity is derived from interactions with auxiliary subunits.1,2 Kv channels and auxiliary subunits participate in the regulation of many cellular processes that form the basis of normal functioning of literally every physiological system. These range from regulation of the heartbeat and neuronal coding to fluid and salt secretion in epithelial tissues such as the kidney and inner ear. Kv channels were also implicated as key regulators of cell fate, either as an obligatory element in the progression of apoptosis3 or as a control element in cell cycle progression and cellular proliferation.4,5 KCNE Subunits Structure and Naming KCNE are membrane-spanning proteins that interact with ion channels and modify their properties. Their structure consists of a single membrane-spanning α helix, and extra- and intracellular N and C termini, respectively (figure 1B). Five human genes were identified and denoted KCNE1-5. The origin of this name stems from the realization that KCNE proteins are Kv channel auxiliary subunits and therefore these proteins are part of the Kv channel superfamily (all human genes of this family begin with KCN). The first isoform to be characterized was KCNE1 (IsK), which was originally named MinK standing for minimal K+ channel. Subsequently, the related isoforms were named Mink Related Peptides or MiRPs.2 Physiological Relevance of KCNE Subunits Several mutations in KCNE genes lead to disease. Long QTs (LQT) are syndromes in which cardiac arrhythmias may occur due to prolongation of the cardiac action potential. This cellular phenomenon is, in many cases, a result of reduction in the magnitude of the K+ efflux, which facilitates repolarization of the cell membrane and action potential termination. In addition to inherited mutations in pore forming Kv channels, mutations in KCNE1 and 2 were found in patients with these cardiac syndromes. Such observations emphasize the importance of KCNE auxiliary subunits in the overall regulation of K+ homeostasis and the cellular mechanisms that depend on it. This is further illustrated by other mutations, causing deafness (KCNE1) or disturbed muscle function (KCNE3).2 The KCNE auxiliary subunits regulate Kv channel Figure 1: A B Extracellular Schematic representation of the Lipid membrane Intracellular 28 C membrane topology of Kvα (A), KCNE(B) and a channel multimer (C). Modulator No.19 Spring 2005 www.alomone.com Expression of KCNE2 in mouse heart Immunohistochemical staining of KCNE2 (MIRP1) in mouse heart, using Anti-KCNE2 (MIRP1) antibody, (#APC-054). Immunoreactivity (red) appeared in striate pattern in heart muscle. activities in many ways, including enhancement or depression of permeation properties and change in gating characteristics. However, in many cases the modulatory action of KCNE on Kv channels is complex, and may combine an impact on the pore and voltage sensing properties.2 Knockout of the KCNE1 gene in mice resulted in phenotype alterations that hint to the involvement of these gene products in cellular volume regulation in epithelial cells,6 a task that is Western blotting of rat heart membranes Western blotting of rat heart membranes 1. Anti-KCNE2 (MiRP1) antibody 1. Anti-KCNE1 (IsK) antibody (#APC-054) (1:200). (#APC-008) (1:200). 2. Anti-KCNE2 antibody, preincubated 2. Anti-KCNE1 antibody, preincubated with the control peptide antigen. with the control fusion protein. common to many K+ channels and is also related to apoptosis.3 KCNE Subunit Interactions By using ion channel toxins and antibodies, the stoichiometry of Kv channel interaction with KCNE subunits was defined as 2 KCNE per functional channel (i.e. a Kv tetramer)7 (figure 1C). In addition, studies pointed out that the KCNE C-terminus (intracellular) interacts with the pore region of the Kv α subunit.8,9 Originally, MinK (KCNE1) was shown to associate with Kv7.1 (KCNQ1 or KvLQT), and this channel’s subunit composition was suggested as corresponding for part of the delayed rectifier K+ current in cardiac tissue.10,11 Indeed, as mentioned before, mutations in both genes result in very similar pathological symptoms (i.e. LQT).1 Subsequently, other known KCNE isoforms, MiRP1 and MiRP2 were shown to modulate other components of the cardiac and skeletal K+ current. MiRP1 (KCNE2) associates and suppresses the other cardiac delayed rectifier component carried out via Kv11.1 (HERG) channels,12 as well as the transient (A type) component associated with Kv4.2.13 MiRP2 (KCNE3) enhances the activity of the skeletal muscle Kv3.4 channel. Mutations in this gene were found in some patients with familial periodic paralysis. It was suggested that disrupted KCNE3 causes reduced K+ currents at KCNE Subunits and Their Interactions with Kv and Kv-like Pore Forming Subunits Gene Main tissue distribution MinK, IsK Expressed in heart, lung, kidney, testis, ovaries, small intestine, and peripheral blood leukocytes. Not detected in pancreas, spleen, prostate and colon. Restrictively localized in the apical membrane portion of epithelial cells Kv3.1: inhibitory- slower activation, stronger inactivation Kv3.2: inhibitory- slower activation Kv4.3: increased current Kv7.1: slower activation, increased conductance Kv11.1: increased current Kv3.1: IP from co-transfected CHO cells Kv3.2: IP from co-transfected CHO cells Kv4.3: electrophysiology Kv7.1: IP from co-transfected Sf9 cells Kv11.1: IP from co-transfected CHO cells Inherited and drug induced LQT, deafness MiRP1 Highly expressed in brain, heart, skeletal muscle, pancreas, placenta, kidney, colon and thymus. A small but significant expression is found in liver, ovary, testis, prostate, small intestine and leukocytes. Very low expression, nearly undetectable, in lung and spleen. Kv3.1: inhibitory- slower activation, stronger inactivation Kv3.2: inhibitory- slower activation Kv4.2: inhibitory but enhances at Vrest due to slow inactivation Kv4.3: increased current Kv7.1: losing voltage dependence: increased current at Vrest Kv11.1: inhibitory HCN1: increased current. HCN2: increased current. HCN4: increased current. Kv3.1: IP from co-transfected CHO cells Kv3.2: IP from co-transfected CHO cells Kv4.2: IP from co-transfected oocytes Kv4.3: electrophysiology Kv7.1: IP from co-transfected COS-7 cells Kv11.1: electrophysiology HCN1: IP from co-transfected oocytes HCN2: IP from cardiomycytes HCN4: Yeast 2 Hybrid Inherited and drug induced LQT (and other cardiac arrhythmias) KCNE3 MiRP2 Widely expressed (skeletal muscle, brain, heart) with highest levels in kidney and moderate levels in small intestine Kv2.1: inhibitory Kv3.1: inhibitory Kv3.2: inhibitory Kv3.4: enhancing current at rest. Kv7.1: losing voltage dependence: increased current at Vrest Kv7.4: inhibitory Kv11.1: inhibitory Kv2.1: IP from rat brain Kv3.1 IP from rat brain Kv3.2: IP from co-transfected CHO cells Kv3.4: IP from co-transfected COS cells Kv7.1: electrophysiology Kv7.4: electrophysiology Kv11.1: electrophysiology Familial periodic paralysis KCNE4 MiRP3 Predominantly expressed in embryo and adult uterus. Low expression found in kidney, small intestine, lung and heart. Kv1.1: inhibitory Kv1.3: inhibitory Kv7.1: slower activation KCNE5 MiRP4 Highly expressed in heart, skeletal muscle, brain, spinal cord and placenta Kv7.1: inhibitory KCNE1 KCNE2 Interaction with channel and effect Identified by Associated disease Protein Kv1.1: electrophysiology Kv1.3: electrophysiology Kv7.1: electrophysiology Kv7.1: electrophysiology AMAE IP = immunoprecipitation. Modulator No.19 Spring 2005 www.alomone.com 29 Expression of Kv4.2 in rat hippocampus Immunohistochemical staining of rat hippocampus with Anti-Kv4.2 antibody (#APC-023). Note that the molecular layers were stained while the mossy fiber terminal region was the other KCNE isoforms had no such an effect on HCN4 channels.20 KCNE proteins are expressed in the brain where KCNE3 was shown to associate with and inhibit the K+ currents carried via Kv2.1 and Kv3.1 channels.22 These results are in line with the observation that Kv3.1 (and Kv3.2) currents are slowed down once the pore-forming gene is coexpressed with KCNE1, KCNE2 or KCNE3.23 When tested for its modulatory activity on Kv1 channels (the Shaker subfamily), KCNE4 was identified as an inhibitory subunit of Kv1.1 and Kv1.3 channels. It had no apparent effect on other Kv1 channels, suggesting that KCNE4 channels specifically interact with these two Kv isoforms.24 Western blotting of rat heart membranes 1. Anti-Kv7.1 antibody (#APC-022) (1:200). 2. Anti-Kv7.1, antibody preincubated with the control peptide antigen not stained. resting membrane potential, leading to membrane depolarization. Interestingly, it was shown that this association reduced the ability of a peptidyl toxin BDS II to block the Kv3.4 currents, hence, demonstrating that the pharmacology of native channel complexes may differ from that of the cloned pore forming α subunit.14 It is interesting to note that while KCNE1 endows Kv7.1 with clear, slow voltage dependent activation10,11 (similar results were obtained with KCNE415 and KCNE516), the association of these pore-forming channels with KCNE217 and KCNE318, results in currents that lack voltage dependency and may serve as “leak” K+ channels. Regarding the specificity of the interactions it should be noted that KCNE5 was found to modulate Kv7.1 currents with no major influence on other Kv7 combinations or on Kv11.1 channels.16 In experimental expression systems, KCNE proteins were shown to interact with and influence a number of Kv and related channels (See Table). The physiological significance of these reported interactions is not always clear and not all of these interactions were detected in vivo. In a number of studies, KCNE2 was implicated as an auxiliary subunit of the Kv related HCN channels.19-21 Hyperpolarization activated channels (HCN) are similar in structure to the archetypal Kv channel. However, while all other voltage dependent channels tend to open in response to membrane potential depolarization, HCN channels open upon membrane potential hyperpolarization. In turn, channel activation results in a net cation influx, which depolarizes the membrane potential, back to rest and beyond. These characteristics equip the cells that express HCN channels with the molecular machinery that is needed for pacing the membrane potential. Hence, the KCNE2 isoform plays a critical role in the generation of cells with pacing capabilities, such as the S-A node in the heart and in pacemaker neurons. It is important to note that 30 KCNE subunits were shown to be an important factor in determining the excitability characteristics and capabilities of cardiac-, skeletal-, smooth-25 muscle and neuronal cells. The patterns of protein associations described above, highlight the necessity of fine-tuning in K+ handling for “proper” physiological functioning. Recently, the expression of several KCNE isoforms was demonstrated in several uterine cancer cell lines.26 In addition, mice carrying a truncation mutation in Kv7.1 channel, the major known partner of KCNE, had increased susceptibility to gastric cancer development.27 Such observations may stress the role played by ion channels in general and KCNE auxiliary subunits in particular, in the cellular mechanisms leading to cancer. Acknowledgments We thank Dr. B. Attalli from The Tel Aviv University for his comments. Inhibition of Kv3.4 channels using BDS-II-a Kv3.4 blocker Inhibition of Kv 3.4 channels expressed in Xenopus oocytes. Left: example traces before (red) and during (black) bath perfusion of 1 µM BDS-II (#B-450). Holding potential was –100 mV, test potential to 0 mV (100 ms) was delivered every 10 seconds. Recordings were made while perfusing ND96 Buffer. Right: time course for the experiment shown on the left. The vertical bar indicates the period of BDS-II perfusion. Modulator No.19 Spring 2005 www.alomone.com References: 22. McCrossan, Z. A. et al. (2003) J. Neurosci. 23, 8077. Anti-Kv3.2 Anti-Kv3.4 Anti-Kv4.2 Anti-Kv4.3 Anti-Kv7.1 23. Lewis, A. et al. (2004) J. Biol. Chem. 279, 7884. 1. Yu, F. H. and Catterall, W. A. (2004) Sci. STKE. 2004, re 15. 24. Grunnet, M. et al. (2003) Biophys. J. 85, 1525. 2. Abbott, G. W. and Goldstein, S. A. N. (2001) Mol. Interven. 1, 95. 25. Ohya, S. et al. (2002) Am. J. Physiol. 282, G277. 3. Meir, A. (2003) Modulator 18, 2. http://www.alomone.com. 26. Suzuki, T. and Takimoto, K. (2004) Int. J. Oncol. 25, 153. 4. Bronstein-Sitton, N. (2002) Modulator 17, 4. http:// 27. Elso, C. M. et al. (2004) Hum. Mol. Genet. 13, 2813. Toxins www.alomone.com. 5. Bogin, O. (2003) Modulator 18, 24. http://www.alomone.com 6. Lock, H. and Valverde, M. A. (2000) J. Biol. Chem. 275, 34849. 7. Chen, H. et al. (2003) Neuron 40, 15. 8. Melman, Y. F. et al. (2004) Neuron 42, 927. 9. Romey, G. et al. (1997) J. Biol. Chem. 272, 16713. Related Products 10. Barhanin, J. et al. (1996) Nature 384, 78. Compound 11. Sanguinetti, M. C. et al. (1996) Nature 384, 80. Antibodies 12. Smith, P. L. et al. (1996) Nature 379, 833. 13. Zhang, M. et al. (2001) Circ. Res. 88, 1012. 14. Abbott, G. W. et al. (2001) Cell 104, 217. 15. Teng, S. et al. (2003) Biochim. Biophys. Res. Commun. 303, 808. 16. Angelo, K. et al. (2002) Biophys. J. 83, 1997. 17. Tinel, N. et al. (2000) EMBO J. 19, 6326. 18. Schroeder, B. C. et al. (2000) Nature 403, 196. 19. Yu, H. et al. (2001) Circ. Res. 88, e84. 20. Decher, N. et al. (2003) Pflugers Arch. Eur. J. Physiol. 446, 633. 21. Qu, J. et al. (2004) J. Biol. Chem. 279, 43497. Anti-HCN1 Anti-HCN2 Anti-HCN4 Anti-KCNE1 Anti-KNCE2 Anti-Kv1.1 Anti-Kv1.3 Anti-Kv11.1 Anti-hKv11.1 Anti-Kv2.1 Anti-Kv3.1b APC-011 APC-019 APC-023 APC-017 APC-050 Product # APC-056 APC-030 APC-052 APC-008 APC-054 APC-009 APC-002 APC-016 APC-062 APC-012 APC-014 rAgitoxin-1 rAgitoxin-2 rAgitoxin-3 BDS-I BDS-II rBeKm-1 rCharybdotoxin α-Dendrotoxin β-Dendrotoxin γ-Dendrotoxin δ-Dendrotoxin Dendrotoxin-I Dendrotoxin-K rErgtoxin-1 E-4031 rHeteropodatoxin-2 rHongotoxin-1 rMargatoxin Phrixotoxin-2 rStromatoxin-1 RTA-150 RTA-420 RTA-390 B-400 B-450 RTB-470 RTC-325 D-350 D-360 D-370 D-380 D-390 D-400 RTE-450 E-500 RTH-340 RTH-400 RTM-325 P-700 RTS-350 Anti- sloβ1 (KCNMB1) sloβ1 is a member of a family of regulatory β subunits tone and hypertension. Studies in sloβ1 knockout mice We are pleased to present a new antibody directed that control the activity of the large conductance Ca2+ - have shown that loss of this subunit results in systemic against the intracellular N-terminus of the rat sloβ1. activated K+ channel KCa1.1. The family includes four hypertension. Conversely, a recent study showed that Anti- sloβ1 (KCNMB1) antibody (#APC-036) will also members with a shared topology: two trans-membrane a commonly found gain-of-function sloβ1 variant has a recognize sloβ1 from human, mouse, dog and rabbit domains, short intracellular N- and C-termini and a protective effect against human diastolic hypertension. origin. large extracellular region. The four members of the family have a distinct tissue distribution with sloβ1 expressed almost exclusively in smooth muscle. Western blotting of rat smooth muscle lysate: 1. Anti- sloβ1 antibody (#APC-036) (1:200). 2. Anti- sloβ1 antibody, preincubated with the control peptide antigen. Functionally, slo β1 increases the sensitivity of the pore-forming KCa1.1 subunit to Ca 2+ and voltage and it also changes its pharmacology. In the past few years there has been much interest regarding the role of the sloβ1 subunit in the regulation of vascular Anti- sloβ4 (KCNMB4) Functionally, slo β4 increases the sensitivity of the Anti- slo β4 (KCNMB4) (#APC-061) will also recognize pore-forming KCa1.1 subunit to Ca2+ and voltage and it sloβ4 from human and mouse origin. also changes its pharmacology. It has been shown that co-expression of sloβ4 with KCa1.1 makes the latter resistant to nM concentrations of the well-known inhibitors Charybdotoxin (#RTC-325) and Iberiotoxin (#RTI-400). The physiological significance of slo β4 expression in the CNS is not clear, but KCa1.1 channels are likely involved in the regulation of neurotransmitter release in presynaptic terminals. We are pleased to introduce a new antibody directed against the intracellular N-terminus of the rat sloβ4. Modulator No.19 Spring 2005 www.alomone.com Western blotting of rat brain membranes: 1. Anti- sloβ4 antibody (#APC-061) (1:200). 2. Anti- sloβ4 antibody, preincubated with the control peptide antigen. 31