* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spatial Reorganization of Glycogen Synthase upon Activation in 3T3
Survey
Document related concepts
Transcript
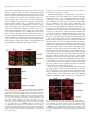
0013-7227/05/$15.00/0 Printed in U.S.A. Endocrinology 146(1):494 –502 Copyright © 2005 by The Endocrine Society doi: 10.1210/en.2004-1022 Spatial Reorganization of Glycogen Synthase upon Activation in 3T3-L1 Adipocytes Hesheng Ou, Limei Yan, Senad Osmanovic, Cynthia C. Greenberg, and Matthew J. Brady Department of Medicine, Committee on Molecular Metabolism and Nutrition, The University of Chicago, Chicago, Illinois 60637 The dephosphorylation of glycogen synthase is a key step in the stimulation of glycogen synthesis by insulin. To further investigate the hormonal regulation of glycogen synthase activity, enzymatic localization in 3T3-L1 adipocytes was determined by immunocytochemistry and confocal microscopy. In basal cells, glycogen synthase and the protein phosphatase1-glycogen-targeting subunit, protein targeting to glycogen (PTG), were diffusely distributed throughout the cell. Insulin treatment had no effect on PTG distribution but resulted in a reorganization of glycogen synthase into punctate clusters. Glycogen synthase aggregation was restricted to discrete cellular sites, presumably where glycogen synthesis occurred. Omission of extracellular glucose or substitution with 2-deoxy-glucose blocked the insulin-induced redistribution of glycogen synthase. Addition of the glycogenolytic agent forskolin after insulin stimulation disrupted the clusters of glycogen synthase protein, restoring the immunostaining pattern to the basal state. Conversely, adenoviral-mediated overexpression of PTG resulted in the insulin-independent dephosphorylation of glycogen synthase and a redistribution of the enzyme from the cytosolic- to glycogen-containing fractions. The effects of PTG on glycogen synthase activity were mediated by multisite dephosphorylation, which was enhanced by insulin and 2-deoxy-glucose, and required a functional glycogen synthase-binding domain on PTG. However, PTG overexpression did not induce distinct glycogen synthase clustering in fixed cells, presumably because cellular glycogen levels were increased more than 7-fold under these conditions, resulting in a diffusion of sites where glycogen elongation occurred. Cumulatively, these data indicate that the hormonal regulation of glycogen synthesis rates in 3T3-L1 adipocytes is mediated in part through changes in the subcellular localization of glycogen synthase. (Endocrinology 146: 494 –502, 2005) G LUCOSE IS PRIMARILY stored as long, branching polymers called glycogen. Glycogen synthesis is regulated by a wide variety of factors including hormones, innervation, activity state, and intracellular metabolites (1). When blood glucose is elevated (i.e. after ingestion of a meal), insulin stimulates glucose storage as glycogen primarily in skeletal muscle but also in liver and adipose tissue. Conversely, during hypoglycemic episodes (i.e. during fasting), liver glycogen stores are mobilized in concert with increased gluconeogenesis to raise hepatic glucose output. Thus, the hormonal modulation of glycogen synthesis and degradation helps maintain plasma glucose concentrations in a narrow, physiological range. However, the molecular mechanisms by which the enzymatic effectors involved in glycogen metabolism are regulated remain poorly understood. Insulin stimulates the storage of glucose as glycogen in muscle and adipose tissue through the coordinate increase in glucose uptake and modulation of glycogen-metabolizing enzymes (1). Insulin binds to its receptor in peripheral tissues and initiates a variety of signaling cascades to increase glucose uptake via translocation of glucose transporter 4 (GLUT4)-containing vesicles to the plasma membrane (2). Glucose enters the cells and is phosphorylated by hexokinases to form glucose-6-phosphate (G6P). Depending on the energy requirements of the cell, G6P can enter glycolysis to generate ATP or be metabolized to uridine diphospho (UDP)-glucose and stored as glycogen. Glycogen synthase, the rate-limiting enzyme for glycogen synthesis, catalyzes the incorporation of UDP-glucose into glycogen chains. Glycogen synthase activity is stimulated by insulin in liver, muscle, and adipose tissue via protein dephosphorylation, allosteric activation, and enzymatic translocation (1). Glycogen synthase is phosphorylated on up to nine residues by a variety of kinases, resulting in its progressive inactivation (3). Insulin increases glycogen synthase activity primarily by stimulating the dephosphorylation of four key residues. Both activation of protein phosphatase-1 (PP1) and inactivation of glycogen synthase kinase-3 (GSK-3) have been proposed to mediate this metabolic effect of insulin (4), although the relative contribution of each enzyme remains unclear. Furthermore, maximal stimulation of glycogen synthase activity by insulin in adipocytes requires increased glucose uptake and metabolism by target cells (5–7). G6P allosterically activates glycogen synthase, overriding phosphorylation-dependent inhibition (6, 8, 9), whereas glycogen content mediates the redistribution of glycogen synthase in hepatocytes, adipocytes, and muscle cells (7, 10, 11). Thus, insulin potently activates glycogen synthase through the coordinate stimulation of glucose uptake and protein dephosphorylation. 3T3-L1 adipocytes are a widely used cellular model for the study of hormonal regulation of glucose metabolism. Ter- First Published Online October 14, 2004 Abbreviations: 2-DG, 2-Deoxy-glucose; FBS, fetal bovine serum; G6P, glucose-6-phosphate; GLUT4, glucose transporter 4; GSK-3, glycogen synthase kinase-3; PP1, type 1 protein phosphatase; PTG, protein targeting to glycogen; PTG-DE, glycogen synthase-binding mutant; UDP, uridine diphospho. Endocrinology is published monthly by The Endocrine Society (http:// www.endo-society.org), the foremost professional society serving the endocrine community. 494 Ou et al. • Regulation of Glycogen Synthase Activity minal differentiation of 3T3-L1 cells into lipid-containing adipocytes results in a dramatic increase in insulin-stimulated glucose uptake and storage as glycogen due to a potent increase in GLUT4 translocation and glycogen synthase activation (12, 13). Previously, we reported that insulin and the glycogenolytic agent isoproterenol exerted opposing effects on glycogen synthase localization in subcellular fractions from 3T3-L1 adipocytes (7, 14), which suggests a potential connection between glycogen synthase activation and intracellular localization in the regulation of glycogen synthesis. In the present study, we have further characterized the regulation of glycogen synthase localization and activation state by insulin, glucose metabolites, and the PP1-glycogentargeting subunit, protein targeting to glycogen (PTG). The results suggest that spatial reorganization of glycogen synthase is involved in the hormonal regulation of glycogen metabolism. Materials and Methods Materials Cell culture reagents and calf serum were supplied by Mediatech, Inc. (Herndon, VA), and fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT.) All other chemicals were from Sigma (St. Louis, MO). UDP-[U-3H]glucose (60 Ci/mmol) was supplied by American Radiolabeled Chemicals (St. Louis, MO). ECL reagent was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden), and GF/A filters were supplied by Whatman (Maidstone, UK). Anti-PTG antibody was generated and affinity purified as described (15). Commercial sources of antibodies were as follows: antiglycogen synthase, Chemicon (Temecula, CA); anti-PP1, Santa Cruz Biotechnology (Santa Cruz, CA); anti-phospho-glycogen synthase (Ser 640) and anti-phospho-GSK-3 (Ser 9), Cell Signaling Technology (Beverly, MA); anti-phospho-acetylconenzyme A carboxylase (Ser 79), Upstate Biotechnology (Charlottesville, VA); horseradish peroxidase-conjugated goat antirabbit and goat antimouse IgG, Bio-Rad (Hercules, CA); and Alexa Fluor 488- and Alexa Fluor 633-labeled secondary antibodies (goat antirabbit and goat antimouse, respectively), Molecular Probes (Eugene, OR). Cell culture 3T3-L1 cells were cultured and differentiated as described (7) and infected within 7 d after completion of the differentiation protocol (15). For immunofluorescence experiments, 3T3-L1 adipocytes were detached by the addition of 0.25% trypsin and replated at an approximate density of 50% onto 15 ⫻ 15-mm no.1 glass coverslips (Carolina Biological Supply, Burlington, NC) in 12-well dishes. Cells were incubated for 24 h to allow cell attachment. Preparation of cellular lysates Cells were washed twice with DMEM supplemented with 25 mm HEPES (pH 7.4), 0.5% FBS, and 5 mm glucose and incubated for 2.5 h in the same medium. The cells were then treated as indicated in the figure legends and washed three times with PBS on ice. For glycogen synthase activity assays and immunoblotting experiments, cells were scraped into homogenization buffer (50 mm HEPES, pH 7.4; 150 mm NaCl; 10 mm NaF; 10 mm EDTA; 10% glycerol; 0.5% Triton X-100; and protease inhibitors added just before use). Lysates were centrifuged for 10 min at 10,000 ⫻ g at 4 C, and supernatants were transferred to new tubes. Cellular fractionation After washing three times with ice-cold PBS, 3T3-L1 adipocytes in six-well plates were scraped into homogenization buffer lacking detergent and lysed by sonication (20% output, 10 sec). All subsequent steps were performed at 4 C. The samples were centrifuged at 1000 ⫻ g for 5 min to pellet nuclei. The supernatants were centrifuged at 10,000 ⫻ g Endocrinology, January 2005, 146(1):494 –502 495 for 15 min. The pellet was saved, and supernatants were then centrifuged at 100,000 ⫻ g for 30 min. The high-speed pellet fraction was termed glycogen-enriched pellet, whereas the final supernatants were saved as the cytosolic fraction. Both pellet fractions were resuspended in detergent-free homogenization buffer using a 23-gauge needle. Immunofluorescence confocal microscopy 3T3-L1 adipocytes plated on glass coverslips were washed two times with ice-cold PBS and then fixed and permeabilized by the addition of ice-cold methanol and acetone (1:1 ratio, vol/vol) for 10 min at ⫺20 C. After fixation, the cells were washed three times with PBS containing 0.5% Tween 20 and incubated for 30 min at room temperature in a blocking solution (5% nonfat dry milk in PBS). Cells were treated by the addition of primary antibodies in a 1:50 dilution for 1 h at room temperature, followed by incubation in labeled secondary antibodies under the same conditions. After washing cells three times with ice-cold PBS, the coverslips were mounted onto labeled slides using nail polish. Confocal microscopy was performed with an Olympus Fluoview 200 Laser Scanning system equipped with a HeNe/Argon laser (Olympus, Tokyo, Japan). Excitation of double-label Alexa 488/Alexa 633 samples was performed with an Argon laser (488 nm) using a 500-nm short pass, dichroic mirror and a HeNe laser (633 nm) using a double dichroic 488/633-nm mirror, respectively. The band pass for Alexa 488 and Alexa 633 emission spectra was set at 500 –575 nm and 600 –700 nm, respectively, with a prism spectrophotometer. The laser rheostat and laser power were both set at 20% for imaging Alexa 488, and both parameters were set at 30% to image Alexa 633. Photomultiplier gain adjustments were set to 50% of maximum level. Optical sections through the z-axis (0.6-m thick; step size, 1.0 m) were collected using a stage galvanometer, and flattened maximum projections of six-section image stacks were generated. Cellular ATP determination 3T3-L1 adipocytes were serum starved for 2.5 h and then washed two times with glucose-free DMEM. Cells were then incubated for 15 min, with or without 100 nm insulin, in DMEM plus 0.5% FBS containing 5 mm of either glucose or 2-deoxy-glucose (2-DG). Cells were washed three times with ice-cold PBS, collected in ATP assay buffer (100 mm Tris, pH 7.75; and 4 mm EDTA), and snap-frozen in a dry ice/ethanol bath. ATP levels were then measured using an ATP Bioluminescence Assay Kit CLS II (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. Other methods Glycogen synthase activity assays and immunoblotting were performed as previously reported (16). Statistical analysis was performed using the Student’s t test. Results Hormonal regulation of glycogen synthase localization in 3T3-L1 adipocytes We previously reported that insulin treatment resulted in the redistribution of glycogen synthase in cellular fractions prepared from 3T3-L1 adipocytes (7, 14). To further investigate the spatial organization of glycogen metabolism in these cells, glycogen synthase localization was determined by immunocytochemistry and confocal microscopy. Fully differentiated 3T3-L1 adipocytes were replated onto cover slips and allowed to recover for 24 h. After a 2.5-h serum starvation, cells were treated for 10 min in the absence or presence of 100 nm insulin. Cells were then fixed, and the coverslips were incubated with the indicated antibodies, followed by fluorescently labeled secondary antibodies. As expected, GLUT4 was primarily located in the perinuclear region in basal cells and translocated to the plasma membrane 496 Endocrinology, January 2005, 146(1):494 –502 after insulin treatment (data not shown). In control cells, both glycogen synthase and the PP1-glycogen targeting subunit PTG were diffusely distributed within cytoplasm and absent from the nucleus (lipid droplets and nuclei appear as dark areas). Insulin had no effect on PTG distribution (Fig. 1A) or PP1 localization (data not shown). In contrast, insulin stimulation resulted in a reorganization of glycogen synthase into bright, punctate clusters (Fig. 1), confirming that the hormone induces enzymatic redistribution in 3T3-L1 adipocytes. Because glycogen synthase translocation in these cells was previously linked to enzymatic activation (14), we presume that these glycogen synthase aggregates formed where de novo glycogen synthesis was occurring. Exposure of basal cells to the glycogenolytic agent forskolin had no effect on glycogen synthase distribution (data not shown). However, forskolin addition to insulin-pretreated cells reversed glycogen synthase clustering (Fig. 1B), further linking the discrete regulation of glycogen synthesis and degradation and the intracellular localization of glycogen synthase. FIG. 1. Hormonal regulation of glycogen synthase (GS) distribution in 3T3-L1 adipocytes. A, Fully differentiated 3T3-L1 adipocytes were trypsinized and replated on glass coverslips. The next day, cells were serum starved for 2.5 h in DMEM containing 5 mM glucose and 0.5% FBS. Cells were then treated without (basal) or with 100 nM insulin for 10 min. After fixation, coverslips were incubated simultaneously with monoclonal anti-GS and polyclonal anti-PTG antibodies and then with secondary antibodies coupled to Alexa Fluor 633 (antimouse; red) or 488 (antirabbit; green). Cells were then analyzed by confocal microscopy. B, 3T3-L1 adipocytes plated on glass coverslips were preincubated for 2.5 h in DMEM containing 5 mM glucose and 0.5% FBS. Replicate wells were treated without (basal) or with 100 nM insulin for 10 min. Half of the insulin-treated cells were washed two times with PBS (37 C) and then stimulated for 30 min with 10 g/ml of forskolin. After fixation, GS localization was analyzed by confocal microscopy. Ou et al. • Regulation of Glycogen Synthase Activity Requirement for extracellular glucose in the insulin-induced redistribution of glycogen synthase Insulin-stimulated glucose uptake plays a critical role in the activation of glycogen synthase in a variety of cell types (reviewed in Ref. 1). To investigate whether the insulininduced glycogen synthase redistribution in 3T3-L1 adipocytes required increased glucose utilization, extracellular glucose conditions were varied. 3T3-L1 adipocytes were seeded on coverslips and serum starved for 2.5 h the next day. Cells were preincubated in medium containing 0 or 5 mm glucose for 15 min before the insulin stimulation. Cells were then fixed and analyzed by confocal microscopy. In the presence of 5 mm glucose, insulin treatment resulted in the formation of glycogen synthase clusters (Fig. 2). However, this insulin effect was completely abolished upon withdrawal of extracellular glucose, suggesting a requirement for a glucose metabolite. Next, cells were preincubated for 15 min in media containing 5 mm 2-DG and then treated with 100 nm insulin. 2-DG is not metabolized after hexokinasemediated phosphorylation, resulting in intracellular accumulation of 2-DG-6-phosphate. Consequently, the substitution of extracellular glucose with 2-DG potentiated glycogen synthase activation by insulin in 3T3-L1 adipocytes (7). Interestingly, insulin did not induce a reorganization of glycogen synthase in cells incubated in 2-DG, indicating that enzymatic activation was not in itself sufficient to promote glycogen synthase translocation. However, acute insulin treatment consistently increased glycogen synthase immunostaining in 3T3-L1 adipocytes incubated in 2-DG-containing media (Fig. 2). The reason for this result is not clear, but it may reflect enhanced recognition of dephosphorylated glycogen synthase by the antibody because total enzyme levels were unchanged in activity assays. Next, the levels of extracellular glucose were varied, and glycogen synthase translocation from the cytosolic fraction was measured by in vitro enzymatic assay and immunoblotting (7). Replicate 12-well plates of 3T3-L1 adipocytes were serum starved for 2.5 h, washed twice in glucose-free DMEM, and then incubated in media containing 0 –5 mm glucose in FIG. 2. Insulin-induced glycogen synthase (GS) redistribution requires increased glucose uptake and metabolism. 3T3-L1 adipocytes were seeded onto coverslips, serum starved for 2.5 h, and then washed two times with PBS (37 C). Cells were then preincubated for 15 min in DMEM plus 0.5% FBS with the indicated glucose addition. After a 10-min treatment in the absence or presence of 100 nM insulin, cells were fixed and GS immunostaining was analyzed using confocal microscopy. Ou et al. • Regulation of Glycogen Synthase Activity the presence of 100 nm insulin. Additionally, some wells were incubated in media containing 5 mm pyruvate in place of glucose. After 15 min, lysates from triplicate wells were pooled and subjected to differential centrifugation to obtain the cytosolic fraction. Glycogen synthase activity was measured in the presence of 10 mm G6P to determine total enzymatic levels. Additionally, lysates were analyzed in parallel by antiglycogen synthase immunoblotting. As shown in Fig. 3, the insulin induced redistribution of glycogen synthase from the cytosolic fraction in the presence of glucose, with a half-maximal effect occurring between 1–2 mm glucose. However, removal of extracellular glucose or substitution with pyruvate completely blocked this effect of insulin. These results confirm a requirement for glucose uptake in the translocation of glycogen synthase in 3T3-L1 adipocytes. 2-DG potentiates the activation of glycogen synthase by PTG Previous studies have shown that the PP1-glycogen-targeting subunit PTG plays an important role in regulation of glucose metabolism by virtue of its ability to bind to glycogen, PP1, and glycogen synthase (17, 18). Adenoviral-mediated overexpression of PTG in 3T3-L1 adipocytes resulted in a marked increase in glycogen synthesis rates and glycogen levels (15). To examine the effects of PTG overexpression on glycogen synthase distribution, 3T3-L1 adipocytes were infected with a recombinant adenovirus encoding PTG. After a 48-h recovery, glycogen synthase activity and localization were determined in replicate wells of cells. As previously reported (15), PTG overexpression markedly increased both the basal and insulin-stimulated glycogen synthase activity ratio without changing total glycogen synthase activity (data not shown). In 3T3-L1 adipocytes, insulin-stimulated glyco- FIG. 3. Cytosolic glycogen synthase (GS) translocation is dependent on glucose uptake. 3T3-L1 adipocytes were serum starved for 2.5 h and then washed twice with glucose-free DMEM. Fresh medium was added with varying amounts of glucose or 5 mM pyruvate in the absence (condition 1) and presence (conditions 2– 8) of 100 nM insulin. After 15 min, cells were lysed in a detergent-free homogenization buffer and subjected to sequential centrifugation at 1,000 ⫻ g and 100,000 ⫻ g. The supernatant from the ultracentrifugation spin was assayed for total GS activity in the presence of 10 mM G6P. Inset, Anti-GS immunoblot from the cytosolic lysates used in the GS activity assay. Conditions: 1, 5 mM glucose; 2, 0 mM glucose; 3, 1 mM glucose; 4, 2 mM glucose; 5, 3 mM glucose; 6, 4 mM glucose; 7, 5 mM glucose; and 8, 5 mM pyruvate. *, P ⬍ 0.05; **, P ⬍ 0.01 vs. basal (condition 1). Results are the average of three independent experiments performed in triplicate. Endocrinology, January 2005, 146(1):494 –502 497 gen synthase activity returned to basal levels in 1–2 h in the continued presence of insulin (14, 19). However, adenoviralmediated overexpression of PTG resulted in a chronic dephosphorylation of glycogen synthase that was maintained for up to 4 d (Fig. 4A), resulting in a 12-fold increase in cellular glycogen levels (data not shown). These data indicated that PTG overexpression overrode the cellular feedback mechanisms that inactivate glycogen synthase activity. The molecular regulation of glycogen synthase activity by the PTG-PP1 complex was further investigated. 3T3-L1 adipocytes were infected with PTG adenovirus, recovered for 2 d, and then stimulated with insulin for 15 min. Additionally, extracellular glucose was substituted with 5 mm 2-DG in half of the wells. Phosphorylation of the key regulatory site 3A on glycogen synthase was analyzed by immunoblotting. Insulin treatment in the presence of 5 mm glucose significantly promoted the dephosphorylation of site 3A, whereas the effects of insulin were enhanced in the presence of 2-DG (Fig. 4B). These data agree with previous work demonstrating that 2-DG potentiates the dephosphorylation of glycogen synthase in adipocytes (5, 7). Interestingly, PTG overexpression alone markedly reduced site 3A phosphorylation to levels below insulin-treated control cells (Fig. 4B). Insulin treatment caused a further reduction in site 3A phosphorylation, although the majority of site 3A dephosphorylation occurred in the absence of insulin in PTG-overexpressing cells. Because dephosphorylation of site 3A is critical for glycogen synthase activation (20), the effects of 2-DG and PTG overexpression on glycogen synthase activity were determined. After infection and recovery, cells were stimulated in the absence or presence of 100 nm insulin for 15 min, in media containing 5 mm glucose or 2-DG. As previously reported, inclusion of 2-DG enhanced basal and insulin-stimulated glycogen synthase activity (Fig. 4C), which is in agreement with the anti-phospho-glycogen synthase immunoblotting data (Fig. 4B). Interestingly, 2-DG also markedly increased glycogen synthase activation by insulin in PTG-overexpressing cells (Fig. 4C). Because site 3A was largely dephosphorylated upon PTG overexpression (Fig. 4B), these results suggest that insulin and 2-DG promote glycogen synthase activation in these cells via the dephosphorylation of other unidentified sites on glycogen synthase. However, it is not possible at this point to completely rule out the further increase in site 3A dephosphorylation as mediating the additional effect on glycogen synthase activation. Next, the potential role of glycogen synthase association with PTG in these effects was determined. 3T3-L1 adipocytes were infected with an adenovirus encoding full-length PTG, containing two alanine substitutions in the glycogen synthase-binding mutant (PTG-DE). Mutation of the aspartic and glutamic acid residues 225 and 228 to alanine in PTG completely blocked glycogen synthase binding to PTG and the stimulation of glycogen accumulation in CHO-IR cells (18) and 3T3-L1 adipocytes (data not shown). Although PTG-DE was overexpressed at comparable levels to PTG (Fig. 4C, inset), the mutant PTG molecule had no effect on glycogen synthase activation under any condition tested. Cumulatively, these results suggest that elevation of intracellular 2-DG-6-phosphate levels by insulin promotes the 498 Endocrinology, January 2005, 146(1):494 –502 Ou et al. • Regulation of Glycogen Synthase Activity FIG. 4. Overexpression of PTG increases glycogen synthase (GS) activity. A, Replicate wells of 3T3-L1 adipocytes were mock infected (M) or infected with PTG adenovirus (P). Half of the wells received 1 g/ml doxycycline (D) during the recovery period to suppress exogenous protein expression (15). Fresh medium was added after 48 h, and cells were collected 4 d after infection. Cell lysates were prepared and analyzed by anti-GS immunoblotting. PTG overexpression resulted in an increase in the electrophoretic mobility of GS, indicative of dephosphorylation and activation. B, Cells were infected as indicated, and 2 d later, they were incubated in the absence and presence of 100 nM insulin for 15 min in media containing 5 mM glucose or 2-DG. Lysates were prepared and analyzed by anti-phospho-GS (pGS) immunoblotting (Ser 640). C, Cells were mock infected or infected with adenoviral constructs encoding wild-type PTG or a full-length PTG construct containing a double alanine substitution in the GS-binding domain (PTG-DE). Cells were allowed to recover for 2 d, and then they were treated for 15 min in the presence or absence of 100 nM insulin in media containing 5 mM glucose or 5 mM 2-DG. GS activity ratios were then determined in vitro; the activity in mock-infected basal cells was set at 100%, which corresponds to an activity ratio of 0.03– 0.07. *, P ⬍ 0.05; **, P ⬍ 0.01 vs. identical condition in the mock-infected cells. Inset, Anti-PTG immunoblots from mock- (M), PTG- (P), and PTG-DE- (DE) infected cells. All immunoblotting results are representative of two to four independent experiments, whereas GS activity measurements are the average of two to five independent experiments. association of glycogen synthase of PTG, resulting in its subsequent dephosphorylation and activation in PTG-overexpressing cells. Effects of 2-DG and PTG on cellular ATP levels Both incubation of cells with 2-DG or adenoviral-mediated overexpression of PTG increased glycogen synthase activity in 3T3-L1 adipocytes. Presumably, both effects occurred by increasing PP1 activity against glycogen synthase, but theoretically, either manipulation could affect cellular ATP concentration and, thus, indirectly result in glycogen synthase dephosphorylation. 2-DG is phosphorylated by hexokinases but does not enter glycolysis to replenish ATP levels. Additionally, there is a marked increase in glucose storage as glycogen upon PTG overexpression, which potentially could be diverting glucose flux into the glycolytic pathway. To further investigate these possibilities, the effects of these experimental conditions on cellular ATP levels were determined. First, serum-starved 3T3-L1 adipocytes were incubated for 15 min with or without 100 nm insulin and in the presence of 5 mm glucose or 2-DG in the extracellular media. Surprisingly, acute inclusion of 2-DG in the absence of insulin markedly reduced ATP levels (Fig. 5A). Furthermore, addition of insulin, which stimulates 2-DG uptake and phosphorylation, caused a greater than 90% drop in ATP levels in 15 min. These results are in contrast to previous work with primary rat adipocytes (5), in which 10 mm 2-DG in the presence of insulin had no effect on ATP levels within 5 min and 1 mm 2-DG had no similar effect on ATP levels for up to 1 h. These differences may reflect a higher rate of glucose uptake and phosphorylation in the cultured 3T3-L1 adipocytes vs. primary cells, resulting in a faster depletion of ATP stores. The effects of the drop in ATP levels were mirrored by an inhibition of insulin-stimulated GSK-3 phosphorylation and a concomitant increase in phospho-acetyl-coenzyme A carboxylase levels (Fig. 5B), which is reflective of cellular energy depletion and AMP kinase activation (21). The addition of 5 or 10 mm pyruvate to the 2-DG-containing media did not reverse these effects (Fig. 5B) or the drop in ATP levels (data not shown), indicating that pyruvate entry into the glycolytic pathway was not sufficient to preserve ATP levels under these conditions of enhanced 2-DG phosphorylation. In parallel, the effects of PTG overexpression on ATP stores were examined. Cells were incubated for 2 d in the absence and presence of PTG adenovirus. Additionally, half of the wells were treated with 1 g/ml doxycycline during the recovery period, which completely suppresses exogenous protein expression (15). PTG overexpression resulted in a trend toward increased ATP levels, although the differences obtained were not statistically significant (Fig. 5C). Thus, the presumed effects of 2-DG on glycogen synthase dephosphorylation in 3T3-L1 adipocytes may involve both increased PP1 activity and a decrease in cellular ATP levels. In contrast, glycogen synthase dephosphorylation upon PTG overexpression appeared to be mediated Ou et al. • Regulation of Glycogen Synthase Activity FIG. 5. Effects of 2-DG or PTG overexpression on cellular ATP levels. A, 3T3-L1 adipocytes were serum starved and washed twice with glucose-free DMEM. Cells were then incubated for 15 min in DMEM containing 5 mM glucose (Glucose) or 5 mM 2-DG in the presence or absence of 100 nM insulin. Cellular ATP levels were determined as described in Materials and Methods. **, P ⬍ 0.01; ***, P ⬍ 0.001 vs. respective condition in glucose-containing media. Results are the average of four independent determinations, each performed in duplicate. B, Cellular lysates were prepared from 3T3-L1 adipocytes that were incubated for 15 min with or without insulin and in the presence of glucose, 2-DG, and pyruvate. Samples were analyzed by anti-phospho-acetyl-coenzyme A carboxylase (pACC) and anti-phospho-GSK-3 (pGSK-3) immunoblotting. Lanes: odd, no insulin; even, ⫹ 100 nM insulin; 1 and 2, 5 mM glucose; 3 and 4, 0 mM glucose; 5 and 6, 0 mM glucose ⫹ 5 mM pyruvate; 7 and 8, 5 mM 2-DG; 9 and 10, 5 mM 2-DG ⫹ 5 mM pyruvate; and 11 and 12, 5 mM 2-DG ⫹ 10 mM pyruvate. Immunoblots are representative of three independent experiments. C, Replicate wells of 3T3-L1 adipocytes were infected as indicated, with 1 g/ml doxycycline (plus dox) added to half of the wells during recovery to suppress exogenous protein expression (15). After 48 h, cells were stimulated for 15 min with or without 100 nM insulin, and cellular ATP levels were determined. Results are the average of three independent experiments, each performed in duplicate. Endocrinology, January 2005, 146(1):494 –502 499 by differential centrifugation. PP1, PTG, and glycogen synthase levels in the fractions were analyzed simultaneously by immunoblotting. In control cells, most of the glycogen synthase protein was localized in the 100,000 ⫻ g and 10,000 ⫻ g fractions, with lesser amounts in the cytosol (Fig. 6). Amylase treatment of these fractions completely released glycogen synthase from the pellets (data not shown), indicating that both fractions contain cellular glycogen. As previously reported, insulin treatment of control cells reduced cytosolic glycogen levels (7), although a corresponding increase in glycogen synthase immunoreactivity in the pellet fractions was not reproducibly detected due to high basal levels. In contrast, most of the PP1 was present in the cytosolic fraction, and its distribution was unchanged by insulin (Fig. 6). Adenoviral-mediated overexpression of PTG significantly elevated PP1 levels in the 100,000 ⫻ g pellet fraction and, to a lesser extent, in the 10,000 ⫻ g pellet fraction. Interestingly, PTG overexpression also resulted in the redistribution of glycogen synthase from the cytosolic- to glycogen-containing fractions (Fig. 4). Thus, PTG overexpression alone appeared to mimic insulin action by increasing glycogen synthase activity (Fig. 4C) and inducing glycogen synthase translocation (Fig. 6), resulting in a dramatic increase in glycogen accumulation (15). To further investigate the effects of PTG on glycogen synthase localization, cells were examined by immunostaining. After infection of 3T3-L1 adipocytes with adenovirus and a 2-d recovery, cells were transferred onto coverslips. The next day, fixed cells were incubated simultaneously with antiglycogen synthase and anti-PTG antibodies, followed by fluorescently labeled antimouse and antirabbit secondary antibodies. PTG levels and glycogen synthase distribution were then determined by confocal microscopy. As shown in Fig. 7, infection of 3T3-L1 adipocytes with PTG adenovirus markedly increased PTG immunoreactivity (Fig. 7, closed arrows). However, because an infection efficiency of roughly 75% was obtained using this viral titer, uninfected cells were present in the same field (Fig. 7, open arrow) and served as a by enhanced PP1 activity and was independent of changes in ATP generation. Overexpression of PTG results in redistribution of glycogen synthase Glycogen synthase localization was examined by subcellular fractionation and immunostaining. 3T3-L1 adipocytes were infected with adenovirus encoding PTG and allowed to recover for 48 h. Cells were serum-starved for 2 h, and half of the cells were treated with 100 nm insulin for 15 min. Cells were collected in detergent-free homogenization buffer and lysed by sonication. Crude cellular fractions were prepared FIG. 6. PTG overexpression induces the redistribution of glycogen synthase (GS) and protein phosphatase-1 (PP1) in 3T3-L1 adipocytes. PTG was overexpressed in 3T3-L1 adipocytes by adenoviral-mediated gene transfer. After a 48-h recovery, cells were serum starved for 2.5 h, followed by treatment without (B) or with 100 nM insulin (I) for 15 min. Cells were collected in detergent-free homogenization buffer and lysed by sonication. Lysates were subjected to sequential centrifugation at 1,000 ⫻ g, 10,000 ⫻ g, and 100,000 ⫻ g. The latter two pellets were resuspended in homogenization buffer using a 23-gauge needle. Samples were then analyzed simultaneously by anti-GS and PP1 immunoblotting. 500 Endocrinology, January 2005, 146(1):494 –502 FIG. 7. Effects of PTG overexpression on glycogen synthase (GS) immunostaining. 3T3-L1 adipocytes were infected in the absence (⫺) and presence (⫹) of adenovirus encoding PTG. Cells were allowed to recover for 48 h before replating onto glass coverslips. The next day, cells were serum starved for 2.5 h before fixation. Coverslips were treated in Fig. 1, and GS and PTG immunostaining of the same cells was examined by confocal microscopy. An infection efficiency of approximately 75% was obtained; infected cells are shown with a closed arrow, and a non-PTG-overexpressing cell is shown with an open arrow. control. Despite the marked activation and redistribution of glycogen synthase after PTG overexpression, there was no detectable clustering of glycogen synthase in infected vs. control cells (Fig. 7). This result was most likely due to the greater than 7-fold increase in cellular glycogen levels during the recovery period (15), which may have resulted in the marked increase in glycogen chains available for elongation and the diffusion of sites within the cell where glycogen synthesis was occurring. Glycogen synthase immunostaining was more intense in the PTG-overexpressing cells, despite no change in total glycogen synthase activity or protein levels (15). As before, this result may be due to differences in antibody recognition of dephosphorylated vs. phosphorylated glycogen synthase. Discussion Insulin potently stimulates glycogen synthesis in muscle and adipose tissue through the synchronized increase in glucose uptake and regulation of glycogen-metabolizing enzymes. After binding of its receptor, insulin activates several signaling cascades that promote the release of GLUT4-containing vesicles from the perinuclear region and translocation and fusion of these vesicles at the plasma membrane (2). In parallel, insulin promotes the dephosphorylation of glycogen synthase and phosphorylase, resulting in enzymatic activation and inactivation, respectively (1). Activation of PP1 by insulin plays an important role in these effects because glycogen synthase and phosphorylase are excellent substrates for the phosphatase in vitro. Additionally, a family of four targeting subunits have been described that bind to PP1 and glycogen, thus targeting the phosphatase to glycogen particles (22). The redundancy of glycogen-targeting subunits in insulin-sensitive tissues suggests an important role for PP1 in the regulation of glycogen metabolism. However, other kinases and phosphatases are likely involved in the regulation of glycogen synthase and phosphorylase activities, so the molecular mechanisms by which insulin controls glycogen-metabolizing enzymes remain uncertain. In addition to regulation by covalent modification, glycogen synthase and phosphorylase activities are modulated by Ou et al. • Regulation of Glycogen Synthase Activity allosteric regulators and changes in intracellular localization (reviewed in Ref. 1). Binding of G6P to glycogen synthase increased enzymatic activity, overriding inhibition caused by phosphorylation (3). Conversely, hepatic phosphorylase activity was suppressed by elevation of intracellular glucose or G6P (1, 23). Interestingly, both hormones and extracellular glucose levels have been reported to alter the subcellular distribution of glycogen synthase (7, 10, 24). In skeletal muscle cells, insulin treatment and increased intracellular glycogen stores caused a clustering of glycogen synthase in single muscle fibers (11). Because glucose transport in hepatocytes is not regulated by insulin, elevation of extracellular glucose alone was sufficient to induce the translocation of glycogen synthase from cytosolic to particulate fractions (1). Immunocytochemistry revealed that glycogen synthase translocated to an area proximal to the plasma membrane, where glycogen synthesis was occurring (25). These effects on hepatic glycogen synthase were mediated by elevations of intracellular G6P levels because they were not blocked by substitution of extracellular glucose with 2-DG (24). Less is known about the regulation of glycogen phosphorylase localization, although the cellular distribution of glycogen may also be affected by hormones and intracellular glucose metabolites (1). In the present study, we found that acute insulin treatment of 3T3-L1 adipocytes induced a redistribution of glycogen synthase into bright, discrete punctate clusters within the cell. Increased glucose uptake and metabolism was required for this effect because withdrawal of extracellular glucose or substitution with 2-DG blocked the change in glycogen synthase immunostaining patterns. Thus, insulin caused a translocation of GLUT4-containing vesicles to the plasma membrane, resulting in enhanced glucose transport and metabolism, and a subsequent translocation of glycogen synthase. Interestingly, sequential treatment of 3T3-L1 adipocytes with insulin and the adenylyl cyclase activator forskolin reverted glycogen synthase immunostaining to the basal state (Fig. 1B). This result indicated that glycogen synthase localization was subject to reciprocal effects of insulin and elevation of intracellular cAMP. We have reported that insulin treatment of 3T3-L1 adipocytes induced the movement of glycogen synthase from cytosolic to denser pellet fractions using sucrose gradients or differential centrifugation techniques (7, 14). Interestingly, the fraction of glycogen synthase that translocated on the sucrose gradients was preferentially activated by insulin (14), suggesting a potential link between enzymatic activation and intracellular redistribution to glycogen. In agreement with reports of skeletal muscle and liver cells (11, 25), we found, in the present study, that glycogen synthesis is apparently restricted to discrete sites within 3T3-L1 adipocytes. Thus, in addition to dephosphorylation and allosteric activation, insulin may regulate glycogen synthase activity in these cells by promoting its localization to elongating glycogen chains. However, further work is required to substantiate this supposition. Substitution of extracellular glucose with 2-DG enhances the activation of glycogen synthase in 3T3-L1 adipocytes (7) most likely through intracellular accumulation of 2-DG-6phosphate (26). Interestingly, 2-DG also markedly increases insulin-stimulated glycogen synthase activity in PTG-over- Ou et al. • Regulation of Glycogen Synthase Activity expressing cells. This effect requires a functional glycogen synthase-binding domain on PTG because overexpression of the glycogen synthase-binding mutant PTG-DE had no effect on glycogen synthase activation. These results suggest that elevation of 2-DG-6-phosphate and, presumably, also G6P might promote glycogen synthase association with PTG, resulting in its dephosphorylation and activation. Moreover, the effect of 2-DG on glycogen synthase activation in PTGoverexpressing cells appeared to involve the dephosphorylation of sites other than 3A on glycogen synthase. Sites 1 and 2A are likely candidates because they have been implicated in the effects of PP1 on glycogen synthase activation, and work is currently underway to test this supposition. However, the marked and unexpected depletion of cellular ATP levels upon acute incubation of 3T3-L1 adipocytes with 5 mm 2-DG clouds interpretation of these results. The drop in ATP was unexpected because normally much higher concentrations of extracellular 2-DG are used to deplete ATP (27). Additionally, previous work in rat primary adipocytes did not find any similar changes in ATP levels upon incubation with 2-DG (5), although the conditions used were not identical. Thus, the enhancement of glycogen synthase dephosphorylation by 2-DG, which was presumed to reflect increased PP1-mediated dephosphorylation, may also involve reduction in ATP levels and, consequently, activity of glycogen synthase kinases in 3T3-L1 adipocytes. In contrast, insulin treatment in the presence of 2-DG did not induce the cellular redistribution of glycogen synthase in 3T3-L1 adipocytes (Fig. 2). This result is in contrast to findings in primary hepatocytes where addition of 2-DG to the medium was sufficient to induce glycogen synthase movement to particulate fractions (24). The reason for the different results is unknown but may reflect differential glycogen synthase isoform expression in the two cell types and/or potentially a requirement for ATP in this effect. However, activation of glycogen synthase by insulin in the presence of 2-DG was not sufficient to induce glycogen synthase movement in 3T3-L1 adipocytes, which is in agreement with previous results (7). Thus, a glucose metabolite downstream of G6P, an ATP-dependent motor protein, and/or glycogen accumulation is required for the intracellular redistribution of glycogen synthase. Additionally, these results suggest that glycogen synthase activation by insulin precedes and can be uncoupled from its translocation to glycogen-containing fractions in 3T3-L1 adipocytes. Activation of PP1 plays an important role in the stimulation of glycogen synthase activity by insulin (28). We have recently reported that adenoviral-mediated overexpression of the PP1-glycogen-targeting subunit PTG in 3T3-L1 adipocytes was sufficient to markedly increase glycogen synthase dephosphorylation and activation (15). In this study, we found that PTG overexpression also induced the translocation of glycogen synthase from cytosolic to particulate fractions in these cells (Fig. 6). Thus, PTG overexpression mimicked the effects of insulin on glycogen metabolism (dephosphorylation of the regulatory site 3A, resulting in stimulation of glycogen synthase activity, enzymatic redistribution, and increased glucose storage as glycogen). Interestingly, these effects occurred without changing basal facilitative glucose transporter number at the plasma membrane (15), indicating Endocrinology, January 2005, 146(1):494 –502 501 that PTG overexpression could pull enough glucose into the cell through existing transporters to sustain the enhanced glycogen synthesis rates. In contrast, PTG overexpression did not cause the distinct clusters of glycogen synthase immunoreactivity that were present after an acute stimulation of 3T3-L1 adipocytes with insulin. However, this result is most likely due to the small changes in glycogen levels after a 15-min insulin stimulation vs. a greater than 7-fold increase in cellular glycogen upon PTG overexpression. This increased number of glycogen chains in PTG-overexpressing cells presumably caused a diffusion of cellular glycogen synthesis from the discrete sites seen in control cells. Similar to reports of skeletal muscle (reviewed in Ref. 29), accumulation of intracellular glycogen reduced glycogen synthase activation by insulin in 3T3-L1 adipocytes (14). Although the mechanism of this feedback inhibition of glycogen synthase activity is unclear, it may represent the spatial separation of glycogen synthase from its insulin-sensitive activators. Interestingly, insulin treatment of 3T3-L1 adipocytes resulted in the redistribution of glycogen synthase away from PTG, as determined by immunostaining (Fig. 1A) and cellular fractionation (data not shown). In insulin-pretreated cells, glycogenolytic agents restored the basal intracellular distribution of glycogen synthase (Fig. 2 and Ref. 14) and subsequent activation by insulin (14). Overexpression of PTG in 3T3-L1 adipocytes resulted in the persistent dephosphorylation and activation of glycogen synthase for up to 4 d and preservation of insulin stimulation, despite a greater than 12-fold elevation of glycogen stores. Thus, elevation of PTG levels overrode the intrinsic negative regulation of glycogen accumulation on glycogen synthase activity, preventing the inactivation of enzymatic activity. Based on this present study and previous work, we propose the following model for the regulation of glycogen synthase activity by insulin in 3T3-L1 adipocytes. Insulin treatment promotes GLUT4 vesicle translocation to the plasma membrane, resulting in an increase in glucose uptake and metabolism. Insulin also stimulates glycogen synthase dephosphorylation through the activation of PP1 and inactivation of glycogen synthase kinases such as GSK-3. The regulation of glycogen synthase activity by the PTG-PP1 complex may be mediated, in part, by elevation of G6P levels in insulinstimulated cells. The enhancement of glucose uptake and metabolism also induces a redistribution of glycogen synthase presumably to elongating glycogen chains. Through an underdetermined mechanism, glycogen accumulation results in the rephosphorylation and inactivation of glycogen synthase and the inhibition of subsequent stimulation by insulin. Subsequent breakdown of glycogen in 3T3-L1 adipocytes restores glycogen synthase distribution to the basal state, priming the enzymes for subsequent stimulation by insulin (7, 14). However, several key issues are still unresolved. What are the relative roles of phosphatase activation vs. kinase inactivation in the insulin-induced dephosphorylation of glycogen synthase? What is the precise nature of the interdependence between glycogen synthase intracellular localization and enzymatic activation? And finally, what are the mechanisms by which increased glycogen levels feed back to inhibit glycogen synthase activity? Further work will be needed to resolve these important issues and determine 502 Endocrinology, January 2005, 146(1):494 –502 whether similar mechanisms regulate glycogen metabolism in hepatocytes and myocytes. Acknowledgments Received August 4, 2004. Accepted October 6, 2004. Address all correspondence and requests for reprints to: Matthew J. Brady, MC1027, 5841 South Maryland Avenue, Chicago, Illinois 60637. E-mail: [email protected]. This work was supported by National Institutes of Health Grant DK64772 and a Career Development Award from the American Diabetes Association (to M.J.B.). Present address for H.O.: Cardiothoracic Surgery, Suite 0200, Baylor College of Medicine, 2450 Holcombe Boulevard, Houston, Texas 77021-2024. References 1. Ferrer JC, Favre C, Gomis RR, Fernandez-Novell JM, Garcia-Rocha M, de la Iglesia N, Cid E, Guinovart JJ 2003 Control of glycogen deposition. FEBS Lett 546:127–132 2. Bryant NJ, Govers R, James DE 2002 Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3:267–277 3. Lawrence Jr JC, Roach PJ 1997 New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes 46:541–547 4. Cohen P, Alessi DR, Cross DA 1997 PDK1, one of the missing links in insulin signal transduction? FEBS Lett 410:3–10 5. Lawrence Jr JC, Larner J 1978 Activation of glycogen synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem 253:2104 –2113 6. Lawrence Jr JC, James C 1984 Activation of glycogen synthase by insulin in rat adipocytes. Evidence of hormonal stimulation of multisite dephosphorylation by glucose transport-dependent and -independent pathways. J Biol Chem 259:7975–7982 7. Brady MJ, Kartha PM, Aysola AA, Saltiel AR 1999 The role of glucose metabolites in the activation and translocation of glycogen synthase by insulin in 3T3–L1 adipocytes. J Biol Chem 274:27497–27504 8. Kato K, Bishop JS 1972 Glycogen synthetase-D phosphatase. I. Some new properties of the partially purified enzyme from rabbit skeletal muscle. J Biol Chem 247:7420 –7429 9. Cadefau J, Bollen M, Stalmans W 1997 Glucose-induced glycogenesis in the liver involves the glucose-6-phosphate-dependent dephosphorylation of glycogen synthase. Biochem J 322:745–750 10. Fernandez-Novell JM, Arino J, Vilaro S, Guinovart JJ 1992 Glucose induces the translocation and the aggregation of glycogen synthase in rat hepatocytes. Biochem J 281:443– 448 11. Nielsen JN, Derave W, Kristiansen S, Ralston E, Ploug T, Richter EA 2001 Glycogen synthase localization and activity in rat skeletal muscle is strongly dependent on glycogen content. J Physiol 531:757–769 Ou et al. • Regulation of Glycogen Synthase Activity 12. Brady MJ, Bourbonais FJ, Saltiel AR 1998 The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3–L1 cells. J Biol Chem 273:14063–14066 13. Summers SA, Whiteman EL, Cho H, Lipfert L, Birnbaum MJ 1999 Differentiation-dependent suppression of platelet-derived growth factor signaling in cultured adipocytes. J Biol Chem 274:23858 –23867 14. Jensen TC, Crosson SM, Kartha PM, Brady MJ 2000 Specific desensitization of glycogen synthase activation by insulin in 3T3–L1 adipocytes. Connection between enzymatic activation and subcellular localization. J Biol Chem 275: 40148 – 40154 15. Greenberg CC, Meredith KN, Yan L, Brady MJ 2003 Protein targeting to glycogen overexpression results in the specific enhancement of glycogen storage in 3T3–L1 adipocytes. J Biol Chem 278:30835–30842 16. Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR 1995 Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem 270:20801–20807 17. Printen JA, Brady MJ, Saltiel AR 1997 PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 275:1475–1478 18. Fong NM, Jensen TC, Shah AS, Parekh NN, Saltiel AR, Brady MJ 2000 Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J Biol Chem 275:35034 –35039 19. Orena SJ, Torchia AJ, Garofalo RS 2000 Inhibition of glycogen-synthase kinase 3 stimulates glycogen synthase and glucose transport by distinct mechanisms in 3T3–L1 adipocytes. J Biol Chem 275:15765–15772 20. Skurat AV, Roach PJ 1995 Phosphorylation of sites 3a and 3b (Ser640 and Ser644) in the control of rabbit muscle glycogen synthase. J Biol Chem 270: 12491–12497 21. Dyck JR, Kudo N, Barr AJ, Davies SP, Hardie DG, Lopaschuk GD 1999 Phosphorylation control of cardiac acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5⬘-AMP activated protein kinase. Eur J Biochem 262:184 –190 22. Newgard CB, Brady MJ, O’Doherty RM, Saltiel AR 2000 Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49:1967–1977 23. Aiston S, Andersen B, Agius L 2003 Glucose 6-phosphate regulates hepatic glycogenolysis through inactivation of phosphorylase. Diabetes 52:1333–1339 24. Fernandez-Novell JM, Arino J, Vilaro S, Bellido D, Guinovart JJ 1992 Role of glucose 6-phosphate in the translocation of glycogen synthase in rat hepatocytes. Biochem J 288:497–501 25. Fernandez-Novell JM, Bellido D, Vilaro S, Guinovart JJ 1997 Glucose induces the translocation of glycogen synthase to the cell cortex in rat hepatocytes. Biochem J 321:227–231 26. Villar-Palasi C, Guinovart JJ 1997 The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J 11:544 –558 27. Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G 2001 Mammalian TOR: a homeostatic ATP sensor. Science 294:1102–1105 28. Brady MJ, Saltiel AR 2001 The role of protein phosphatase-1 in insulin action. Recent Prog Horm Res 56:157–173 29. Holloszy JO, Kohrt WM, Hansen PA 1998 The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 3:D1011–D1027 Endocrinology is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the endocrine community.