* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download teaching and learning materials - UNESDOC
Inorganic chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
History of electrochemistry wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Water pollution wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Click chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Biochemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Acid strength wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Organic chemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Petasis reaction wikipedia , lookup
Acid–base reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
SC/BES/MCS/2006/13 December 2006 Original: English Organic Chemistry Microscience Experiments Teaching and Learning Materials MANUAL FOR TEACHERS Compiled by Beverly Bell & Christopher Gunter Edited by Prof. JD Bradley and Jane Spriggs © 2006 RADMASTE Centre µscience The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre University of the Witwatersrand, Johannesburg Prepared under UNESCO Contract No. 4500021563 This Booklet of Organic Chemistry Microscience Experiments has been Prepared in Cooperation with UNESCO, IOCD and IUPAC UNITED NATIONS EDUCATIONAL, SCIENTIFIC AND CULTURAL ORGANIZATION INTERNATIONAL ORGANIZATION FOR CHEMICAL SCIENCES IN DEVELOPMENT INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY – IUPAC IN COLLABORATION WITH µscience THE UNESCO-ASSOCIATED CENTRE FOR MICROSCIENCE EXPERIMENTS THE RADMASTE CENTRE UNIVERSITY OF THE WITWATERSRAND JOHANNESBURG, SOUTH AFRICA THE INTERNATIONAL FOUNDATION FOR SCIENCE EDUCATION JOHANNESBURG, SOUTH AFRICA SESI SCIENCE EDUCATION SOLUTIONS INTERNATIONAL P.O. BOX 681, WITS 2050, JOHANNESBURG, SOUTH AFRICA Worksheets Prepared By : Ms B. Bell & Mr C. Gunter (UNESCO-Associated Centre for Microscience Experiments, The RADMASTE Centre, University of the Witwatersrand) µscience Worksheets Edited By : Prof. J. Bradley (International Foundation for Science Education); Ms J. Spriggs (The RADMASTE Centre) Introductory Note to Teachers The Organic Chemistry Microscience experiments in this book have been designed for use with an upgraded RADMASTE Advanced Microchemistry Kit. Users of the Advanced Microchemistry Kit can acquire an Organic Microchemistry Accessories Kit to upgrade their kits. It contains the required additional items of equipment and is available from the RADMASTE Centre at low cost. Organic Chemistry Microscience Experiments FOREWORD 7 INTRODUCTION 8 Components of the RADMASTE Organic Microchemistry Kit 9 CHAPTER 1 10 Qualitative Testing of Organic Compounds 1. Preparing Esters 2. Tests for Aldehydes and Ketones 3. The Iodoform Reaction 4. Tests for Identifying and Classifying Alcohols 30 5. Tests for Unsaturated Hydrocarbon Compounds 40 6. Investigating Intermolecular Forces in Organic Compounds by Measuring the Boiling Point 48 Melting Point Determinations 51 7. 12 17 25 CHAPTER 2 53 General Procedures 1. 2. Assembling the Wire Microvial-holder Vacuum Filtration and Washing of the Collected Solid 55 56 3. 4. 5. Making Capillary tubes for Melting and Boiling Point Determinations General Procedure for Boiling Point Determinations General Procedure for a Melting Point Determination 58 58 60 6. 7. Making TLC Spotting Capillary Tubes General Procedure for a Thin Layer Chromatogram (TLC) 62 63 The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za CHAPTER 3 65 Preparation and Reaction of Organic Compounds 1. 2. 3. 4. 5. 6. Preparation and Testing of Ethyne (Acetylene) Making Esters – the Synthesis of Aspirin Recrystallisation and Purification of Aspirin The Oxyacetylene Flame Acid-base Extraction of Benzoic Acid from Naphthalene Using Esters: Saponification The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 67 72 76 78 81 85 FOREWORD The International Organisation for Chemical Sciences in Development (IOCD) was established more than 22 years ago. Since its inception, it has been assisting its partners in many different parts of the world - with special emphasis on the developing countries to create and develop projects on chemical sciences. Professor Glen Seaborg and I, together with all our colleagues, have always tried to find the best solutions during the development stages of such projects. We have worked with the different Intergovernmental (IGO) and Non-governmental (NGO) organisations, and especially with UNESCO - the unique organisation of the United Nations system with the international mandate to develop programme activities within the basic science disciplines and mathematics. Amongst these the chemical sciences have been described as the "essential science" by Professor Glen Seaborg. Today we are very pleased to present to the world community, the new teaching and learning packages on Organic Chemistry Microscience Experiments. These materials can be considered as an extension of the previous Advanced Teaching and Learning Packages which use microchemistry activities to address general and inorganic chemistry. The Microchemistry Packages have been published by UNESCO in different languages, and have recently become available on the Internet for free access and use by UNESCO member states. The organic chemistry materials open up new possibilities of using microscience kits at different educational levels to do practical laboratory work. In some countries, the educational curriculum allows for organic chemistry at secondary school level to be part of a general chemistry syllabus; in some other countries, organic chemistry is an independent part of chemical sciences at secondary level. Whatever the case may be, these educational materials will provide simple and cost-effective methods to do new practical laboratory work with the microscience kits. We hope, too, that these Organic Chemistry Microscience Experiments will soon be installed on the Internet for free use. Some years ago, Professor John Bradley, President of the International Foundation for Science Education (IFSE), Director of the RADMASTE Centre, and formerly Chairman of IUPAC-CTC, received the Pierre Crabbe IOCD Award for outstanding contributions to the advancement of science education in the developing countries. In his introductory statements which follow, you can find some concrete details on the new educational materials contained in this publication. Finally, please let me express my hope that these new microscience materials will be successfully used in the different countries of the Globe, and that this will lead to different language versions being prepared so that all may have access to practical organic chemistry. Professor Jan Marie Lehn President, IOCD Nobel Prize winner The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 7 INTRODUCTION All over the world, science educators declare that practical experiences are an essential part of learning science. However, in many countries these experiences are not provided in the majority of their primary and secondary schools. There are several reasons for this: cost, safety, waste disposal and teacher preparation. In the last few years a global programme under the aegis of UNESCO, and in association with a number of organisations and donors, has attempted to address and overcome that situation. The programme has promoted the idea that practical experiences can be provided at much lower cost and with much lower environmental impact by working on a small scale and using less expensive materials. The programme has had much success. When the idea has been introduced (so far in more than 70 countries) it has always had a positive reception. In several countries, local initiatives have taken the idea further through pilot projects and on to wider national implementation. This programme of introducing the concept is continuing. This satisfying record is however not enough. Not surprisingly, the original repertoire of experiences and low-cost equipment designs, has some limitations. In chemistry, for example, major areas of the subject have been ignored. One of these is organic chemistry. In several countries, the welcome accorded to the basic ideas, has been coupled with questions about these important missing areas. This workbook responds to these questions, by offering a limited repertoire of basic organic chemistry experiences that can be provided with low-cost small scale equipment. They have been developed at the RADMASTE Centre, University of the Witwatersrand in South Africa, the Centre which created the original materials referred to above. The worksheets and teacher guide layout will therefore be familiar to those who have used the previous chemistry books, which were published by UNESCO. Much of the equipment used will also be familiar, but there are some new and modified items. In modern laboratories around the world, chemistry is increasingly done on the small scale. Organic chemistry has been one of the leaders in this trend and it is therefore fitting that this new publication offers experiences in this field to those who are starting their studies of it. We hope that both teachers and learners will enjoy the experiments, and will improve and modify them as a result of their own experience. Although many of the experiments can be carried out at the secondary school level, it is suggested that some of the activities be attempted by tertiary level students only. Professor JD Bradley Director, RADMASTE Centre & UNESCO Associated Centre for Microscience Experiments The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 8 R AD CE MAST E N T RE COMPONENTS OF THE RADMASTE ORGANIC MICROCHEMISTRY KIT silicone tube microspatula gas collecting tube lid 1 plastic forceps lid 2 propette 1 2 wooden toothpick 4 3 5 6 7 8 9 10 11 12 A string B C microfunnel D wire vial-holder E fusion tube F C 5 0 4 0 100 glass rod 8 0 1 2 3 4 6 5 Organic comboplate7 2,5 2 0 6 0 4 0 2 0 0 0 combustion tube pH 1-4 acid 5 6 7 neutral 8 9 10 11-14 basic pH indicator chart alcohol thermometer glass vial with lid organic vial syringe microburner microstand 9 CHAPTER 1 Qualitative Testing of Organic Compounds 10 Qualitative Testing of Organic Compounds 1. Preparing Esters 12 2. Tests for Aldehydes and Ketones 17 3. The Iodoform Reaction 25 4. Tests for Identifying and Classifying Alcohols 30 5. Tests for Unsaturated Hydrocarbon Compounds 40 6. Investigating Intermolecular Forces in Organic Compounds by Measuring the Boiling Point 48 Melting Point Determinations 51 7. 11 PREPARING ESTERS TEACHER'S GUIDE AND MODEL ANSWERS INTRODUCTION Esters are a group of organic compounds which can be made by reacting an alcohol with a carboxylic acid in the presence of a sulphuric acid catalyst. The formation of an ester - or esterification - is an example of a general class of reactions called condensations. The condensation reaction results in the formation of water as the ester is formed. The general reaction equation is: O R1OH + R2COH O R2C O R1 + H2O R1 and R2 are hydrocarbon groups that may be the same or different. Esters have characteristic odours that differ substantially from the sometimes foul smelling alcohols and carboxylic acids from which they are derived. Many of the pleasant odours of fruits and flowers are due to the presence of low molecular weight esters. Both the pharmaceutical and food industries use esters to enhance the flavours and fragrances of various products. For example, benzyl ethanoate is the oil of jasmine used in perfumes while pentyl ethanoate contributes to the pear fragrance of pear drop sweets. 1. Chemicals All the chemicals required for the esterification reactions are found in the requirements table preceding the experiment. If the organic acids and alcohols required are not available, others can be used. Some examples are isoamyl alcohol reacted with ethanoic acid, ethanol reacted with benzoic acid, pentanol reacted with butanoic acid and methanol reacted with butanoic acid. Consult relevant textbooks for further combinations of alcohols and acids. 2. Apparatus The propettes, microspatula, glass rod, microburner and matches are all components of the RADMASTE Microchemistry kits. The organic comboplate7 is manufactured from a material which is resistant to organic solvents and must be used in this experiment, because the conventional comboplatewill be dissolved by the organic acids and most of the alcohols. A scissors is an optional requirement for cutting a piece of paper towel into smaller squares. Students may tear the towelling into smaller pieces instead. Gloves should be worn throughout the experiment to protect the skin from the corrosive nature of the chemicals used. The teacher must carry out a risk assessment to decide whether protective glasses should be worn by the students. If the teacher is not comfortable using the open flame of a microburner in the vicinity of organic substances, a water bath may be set up by pouring boiling water into a plastic container. This option is reliant on the availability of apparatus for boiling water, such as an electric kettle, and plastic containers must be large enough for a comboplate7 to fit into. The teacher must also ensure that a waste container is provided into which all organic waste can be disposed of. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 12 3. Hints Since all the chemicals used in the ester preparations are colourless, it is a good idea to advise students to label their propettes prior to the experiment to prevent these from becoming muddled. Two or three students can share one propette of alcohol/acid per ester made because only three or four drops of each liquid is required. The same applies to the use of the concentrated sulphuric acid. When heating the glass rod, it must not be held for a long time in the flame of the microburner as this will cause the glass to become too hot. The liquid mixture in the wells will be boiled. Since many of the alcohols have low boiling points, they may evaporate quickly and the experiment will have to repeated. The end of the glass rod must be waved five or six times through the flame for the best results. Stirring the glass rod through contents of the well will allow the heat to be distributed throughout the mixture. The gentle immersion heating should be repeated three times to ensure that the ester is formed. If a boiling water bath is used, the boiling water should be poured into a plastic container just prior to heating the comboplate7 , which must then be floated in the water bath for about three minutes for complete esterification. Care must be taken not to push the comboplate7 into the water because the wells will be flooded. Results with the water bath are normally better than with immersion heating as the odours of the different esters are more evident. However, use of a water bath requires replenishment of the boiling water from time to time and takes slightly longer than the immersion process. If the teacher is confident that the students are skilled enough to carry out the experiment without all the step-by-step instructions, then a table such as the one shown below could be provided after step 11 in the procedure. Quantity of Alcohol Quantity of Organic Acid Methanol and salicylic acid (Well A3) 5 drops 1 small spatula using narrow end of microspatula Pentanol and ethanoic acid (Well A5) 5 drops 5 drops Isobutanol and formic acid (Well A7) 5 drops 5 drops Mixture/Well Quantity of H2SO4(aq) Heating 3 x immersion heatings with glass rod or 3 minutes in water bath 1 drop As above As above Students will still need instructions for the smelling of the starting materials and resulting esters to be able to complete the results table. Each well should be dried out with paper towel after the odour of the ester has been identified. This is especially necessary when using a water bath for heating, as the odours of the different esters may mix and the student may not be able to detect the characteristic odour of a specific ester. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 13 During the formation of pentyl ethanoate and isobutyl formate, the colour of the liquids may become yellow. This is not part of the esterification reactions. Similarly, the formation of white crystals during synthesis of methyl salicylate is also not part of the esterification. The predict, explore, explain exercise for showing the catalytic function of the sulphuric acid can be incorporated into the main experiment if liked. Try to get students to each bring an empty container or label of one food item and one cosmetic product. Let them examine the list of ingredients and identify which of these are esters. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Do not inhale the acids and alcohols deeply! The vapours are easily detectable. Serious respiratory damage and dulled receptiveness may occur if this rule is not adhered to. In particular, the glacial acetic acid, formic acid and isobutanol have sharp, burning odours that also irritate the eyes. Move all organic reagents away from the flame when using the microburner. They are flammable! Concentrated sulphuric acid is extremely corrosive. If any acid is spilt on the skin, the affected area must immediately be rinsed with copious amounts of water. Severe burns must receive medical attention. Never point a propette or a syringe containing acid upwards. A momentary lapse of concentration can result in a nasty accident. If any acid is squirted into the eye, immediately rinse the eye out under running water. Always have a dilute solution of sodium hydrogencarbonate (household baking soda), or milk close by to apply to the injury. These substances will help neutralise the acid in the eye. The patient should be referred to a doctor. Dispose of all organic waste in the waste container provided, and not down the drain! The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 14 MODEL ANSWERS TO QUESTIONS Q1. Complete Table 1 by naming each of the esters formed during the reactions. A1. TABLE 1: DESCRIPTION OF THE ODOURS OF EACH ALCOHOL, CARBOXYLIC ACID AND THE RESULTING ESTER WHEN THESE ARE REACTED ALCOHOL AND DESCRIPTION OF ODOUR CARBOXYLIC ACID AND DESCRIPTION OF ODOUR Ethanol Glacial acetic acid/Ethanoic acid Smells like alcohol in alcoholic beverages e.g cane Strong smell of vinegar Methanol Salicylic acid Similar to methylated spirits Odourless Pentanol/Amyl alcohol Glacial acetic acid/Ethanoic acid Sweet smelling Strong smell of vinegar Isobutanol/2 methyl propanol Formic acid Sharp, burning odour Sharp burning odour DESCRIPTION OF ODOUR OF PRODUCT NAME OF ESTER Smells like nail polish remover Ethyl ethanoate or ethyl acetate Smells like oil of wintergreen Methyl salicylate Smells like pear (e.g in pear drop sweets) Pentyl ethanoate or pentyl acetate or amyl acetate Smells fruity - similar to raspberry Isobutyl formate or 2-methylpropyl formate Q2. What is the name given to the type of reaction by which esters form from a carboxylic acid and an alcohol? A2. An esterification reaction. Q3. What evidence is there that esters formed in the reactions studied? A3. Students should identify that the ester smells more pleasant than the original reactants. Q4. Complete the following predict, explore, explain exercise to determine the role of the sulphuric acid in each of the reactions: PREDICT Will an esterification reaction take place in the absence of concentrated sulphuric acid? The students must make their own predictions and test these by completing the procedure below. A4. The correct prediction is that no ester can be formed without the concentrated H2SO4(aq). EXPLORE Has the expected ester been formed? How do you know this? No. The odour is that of sour vinegar due to the presence of glacial acetic acid. There is no smell of the expected ethyl ethanoate (nail polish remover). The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 15 EXPLAIN Was your prediction correct? This answer will depend on the original prediction made. What is the role of concentrated sulphuric acid in the esterification reaction? Concentrated sulphuric acid catalyses the esterification reaction. Without it, an ester forms very slowly. Q5. The introduction to this experiment shows the general equation for an esterification reaction. Use the equation as a guide to write down balanced equations representing each of the esterification reactions you have performed. The formulae of each alcohol and carboxylic acid are given under the chemical requirements. If possible, use a different colour pen to show the alcohol. A5. a) Ethanol and acetic acid: C2H5OH + CH3COOH → C2H5OOCCH3 + H2O b) Methanol and salicylic acid: CH3OH + C6H4OHCOOH → CH3OOCOHC6H4 + H2O c) Pentanol and acetic acid: C5H11OH + CH3COOH → C5H11OOCCH3 + H2O d) Isobutanol and formic acid: (CH3)2CHCH2OH + HCOOH → (CH3)2CHCH2OOCH + H2O Q6. Using the description of the odours of each ester you have prepared in this experiment, choose two industries in which you think esters are used commercially. Give reasons for your answers. A6. a) The food industry, because some esters like pentyl ethanoate and isobutyl formate have a fruity aroma that can be used to enhance the flavour of certain foods. b) The cosmetic industry, because some esters like methyl salicylate and ethyl ethanoate may be used as fragrances for creams, gels and other cosmetic products. Q7. If possible, try and obtain the containers of some food and cosmetic products. Read the lists of ingredients and identify any esters which appear on the labels. A7. There are a number of examples. One common ester in food products is monosodium glutamate (MSG), which is used as a flavour enhancer. Benzyl acetate (benzyl ethanoate) is the oil of jasmine in perfumes. Q8. Do all ethyl esters have the same smell? Do all formates have the same smell? Design, carry out and report on a piece of research to answer these questions. A8. Students should react ethanol with salicylic acid and formic acid to show that ethyl salicylate, ethyl formate and ethyl ethanoate smell different. The second investigation would require the reaction of pentanol and methanol with formic acid. The pentyl formate and methyl formate have different odours, as does the ethyl formate already synthesised. This is because the smell of an ester is related to its structure and since all the esters have different structures, they all smell differently. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 16 FUNCTIONAL GROUP ANALYSIS Tests for Aldehydes and Ketones TEACHER'S GUIDE AND MODEL ANSWERS Part 1: The 2,4-Dinitrophenylhydrazine Test for Aldehydes and Ketones The molecules of aldehydes and ketones contain the carbonyl, -C=O, group. Other organic compounds such as carboxylic acids and their derivatives, also contain the carbonyl group as part of their molecular structure. The molecules of compounds like alcohols contain the -C-O- group. It is therefore important to be able to distinguish ketones and aldehydes from these other carbonyl and oxygen containing compounds. In this experiment you will mix an aldehyde, a ketone, an alcohol, an ester and a carboxylic acid with a solution called 2,4-dinitrophenylhydrazine (2,4-DNP) reagent. The reactions of each of these compounds with the 2,4-DNP will be used to differentiate the aldehyde and ketone. Part 2.1: Reduction of Ammoniacal Silver Nitrate Solution - Tollen’s Test The 2,4-DNP test can help the chemist establish which substances are aldehydes and/or ketones from a variety of carbonyl containing compounds. However, it cannot be used to determine which of these substances are the aldehydes and which are the ketones because both classes of compounds form precipitates with the 2,4-DNP reagent. In this experiment, the differing reactions of aldehydes and ketones with the Ag(NH3)2+(aq) ions in ammoniacal silver nitrate solution (Tollen’s reagent) will distinguish an aldehyde from a ketone. Part 2.2: Schiff’s Test to Distinguish between Aldehydes and Ketones Schiff’s reagent contains a dye compound which has been decolourised by sulphur dioxide. The colourless compound is unstable and will yield a violet-purple colour when reacted with certain substances. The task in this experiment is to determine how the colour reaction with Schiff’s reagent can be used to distinguish between aldehydes and ketones. 1. Chemicals Part 1: The 2,4-Dinitrophenylhydrazine Test for Aldehydes and Ketones All the chemicals required for Part 1 are found in the requirements table preceding the experiment. The 2,4-dinitrophenylhydrazine reagent (Brady’s reagent) can be prepared by suspending 2.0 g of solid 2,4-dinitrophenylhydrazine in 100 ml of methanol. To dissolve the solid, 4.0 ml of concentrated sulphuric acid must be added cautiously and slowly. If the solution is not clear, filtering may be necessary. This volume of reagent should be sufficient for an entire class. If the methanol, formic acid and ethyl acetate are not available, then another alcohol, carboxylic acid and ester may be used to show that 2,4-DNP does not react with these compounds. It is not essential that formaldehyde and propanone are used as the aldehyde and ketone to be tested. Other aliphatic aldehydes and ketones are also suitable. (For the purpose of providing model answers, the results of the 2,4-DNP test with butyraldehyde have also been supplied.) Glacial acetic acid may be needed for removing solid residue from the wells of the comboplate7 after the experiment. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 17 Part 2.1: Reduction of Ammoniacal Silver Nitrate Solution - Tollen’s Test All the chemicals required for Part 2.1 are found in the requirements table preceding the experiment. The 0.3 M silver nitrate solution can be prepared by dissolving 5.096 g of solid silver nitrate in a little distilled water in a volumetric flask, and then making up the volume to 100 ml. Other aliphatic aldehydes and ketones can be mixed with the ammoniacal silver nitrate solution, but aromatic ketones are not recommended for testing because these - together with α-hydroxyketones and α-diketones - reduce Tollen’s reagent and give a positive result. Caution should be exercised when testing unknown compounds with Tollen’s reagent as some other reducing substances like hydroxylamines, and salts and esters of formic acid can also produce a silver mirror. Part 2.2: Schiff’s Test to Distinguish between Aldehydes and Ketones All the chemicals required for Part 2.2 are found in the requirements table preceding the experiment. Schiff’s reagent is readily available from commercial suppliers, but can be prepared by dissolving 2 g of sodium bisulphite in a solution of 0.2 g of p-rosaniline hydrochloride (fuchsin) and 2 ml of hydrochloric acid in 200 ml of distilled water. The reagent is susceptible to decomposition by light, heat, alkalis and alkali salts. It should therefore be kept in a well stoppered bottle and protected from exposure to heat and light. If the bottle is not adequately stoppered, the solution will gradually lose sulphur dioxide and the purple colour will return. As in the previous parts of the experiment, other aliphatic aldehydes and ketones can be tested with Schiff’s reagent. The result of this test with butyraldehyde has been provided in the model answers, together with that for formaldehyde. The use of aromatic aldehydes and ketones should be avoided as some aromatic aldehydes in particular, give a negative result with Schiff’s reagent. 2. Apparatus The propettes and microspatulas are components of the RADMASTE Microchemistry kits. The organic comboplate® is manufactured from a material which is resistant to organic solvents and must be used in this experiment, because the conventional comboplate® will be dissolved by the aldehyde, ketone, carboxylic acid and ester. Gloves should be worn throughout the experiment to protect the skin from the corrosive nature of the chemicals used. The teacher must carry out a risk assessment to decide whether protective glasses should be worn by the students. The teacher must also ensure that a waste container is provided into which all organic waste can be disposed of. 3. Hints Since most of the chemicals used in all parts of the experiment are colourless, it is a good idea to advise students to label their propettes prior to the experiment to prevent these from becoming muddled. Two or three students can share propettes of the 2,4-DNP reagent, the Schiff’s reagent, formaldehyde, propanone etc. because only a few drops of each is required. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 18 Part 1: The 2,4-Dinitrophenylhydrazine Test for Aldehydes and Ketones The aim in Part 1 is to show that the 2,4-DNP test can be used to distinguish aldehydes and ketones from other compounds whose molecules also contain an oxygen atom bonded to a carbon atom, as well as from other carbonyl-containing compounds. For this reason, the procedure requires that an alcohol, a carboxylic acid and an ester (a carboxylic acid derivative) are mixed with the 2,4-DNP reagent. Only the ketone and aldehyde will react to form 2,4-dinitrophenylhydrazones. Formaldehyde reacts immediately with the 2,4-DNP in the absence of agitation. Propanone, like other ketones, requires stirring to ensure mixing of the ketone and the 2,4-DNP. A crystalline derivative is observed after a few minutes. Other aldehydes and ketones, such as butyraldehyde, will react with the 2,4-DNP but require further agitation to induce crystallisation. A standing period is therefore allowed for in the experiment, after which stirring may still be required before the formation of crystals is observed. The red, yellow or orange precipitates should be washed immediately from the wells of the comboplate® as soon as Part 1 has been completed. Staining may occur if the precipitates are left for a prolonged period of time in the wells. Boiling water can be used to flush the precipitates from the wells. If the stains have still not been removed, a few drops of glacial acetic acid can be added to each affected well. The stains can then be rubbed off using a cotton bud, or the pointed end of a wooden skewer around which a small strand of cotton wool has been wrapped. Part 2.1: Reduction of Ammoniacal Silver Nitrate Solution - Tollen’s Test When preparing the “Tollen’s reagent” in the large wells of the comboplate®, brown silver oxide (Ag2O(s)) is formed by adding sodium hydroxide solution to the aqueous silver nitrate. This precipitate must be dissolved with the ammonia so that [Ag(NH3)2]+ ions can be formed in solution. An excess of ammonia must be avoided, therefore the ammonia solution must be added dropwise with stirring. Sometimes it may appear that the silver oxide has not completely dissolved, but if stirring is continued for a short while all the small, brown particles of silver oxide will eventually be dissolved. No more than 25 drops of 1 M NH3(aq) should be added. The number of drops of aldehyde added to the reagent varies between 1 and 3, depending on the aldehyde used. Only one drop of formaldehyde is required to form a conspicuous silver mirror. The silver mirror formed with butyraldehyde is however not as evident when one drop of the aldehyde is used. Three drops of this aldehyde is preferable if a better silver mirror is desired. A waiting period of five to ten minutes is required to allow the silver precipitate to accumulate on the bottom and sides of the well. Formaldehyde reacts immediately and the silver mirror is normally detected before five minutes has lapsed. Other aldehydes, like butyraldehyde, react more slowly and the silver is only evident after some time. If another aldehyde is used by the teacher and a silver mirror is not observed after 10 minutes, the comboplate® should be floated in a container of warm - but not boiling - water (approximately 350C). A silver mirror should form within a few minutes. The appearance of the silver mirror varies with the aldehyde used. For example, the mirror formed with formaldehyde is very shiny whereas that formed with butyraldehyde is somewhat darker. It is usually necessary to tilt the comboplate® towards the light to show the silver deposit on the sides of the wells. The silver solutions should never be heated excessively, nor left to stand unnecessarily since explosive silver fulminate may be formed. Once the experiment has been completed, the residue from each well must be thoroughly rinsed way. Dilute nitric acid must then be added to each well to remove any silver mirror and prevent the formation of fulminates. Sometimes there is still some black residue that remains in each well after washing with nitric acid. This can be removed with a cotton bud or wooden skewer as previously described. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 19 Part 2.2: Schiff’s Test to Distinguish between Aldehydes and Ketones Schiff’s reagent is very easily decomposed by heat, light, alkali, alkali salts of weak acids and when exposed in small quantities to the air for some time. Mineral acids also reduce the sensitivity of the test. As a result the Schiff’s reagent becomes pale purple in colour, which darkens with further deterioration of the solution. If the teacher finds that the Schiff’s reagent is no longer colourless but already pale purple in colour prior to performing the experiment, the following procedure should be used: Place 10 drops of the reagent in wells A1, A2 and A3. Place 1 drop of the aldehyde in well A2 and 1 drop of the ketone in well A3. The reagent in well A1 will be used as a colour comparison. The aldehyde will react with the reagent to produce a darker purple colour within two minutes. The ketone will not react and the colour in well A3 will be the same as the control in well A1. The student will still be able to deduce that the aldehyde gives a positive result with Schiff’s reagent, whereas that with the ketone is negative. If the Schiff’s reagent has deteriorated to such an extent that the solution is dark purple in colour, it cannot be used and should be discarded. Students must be advised to keep the bottle containing the Schiff’s reagent closed at all times. Since some compounds may contain traces of other substances that react with Schiff’s reagent, a time limit of two minutes has been set in this experiment for observing the change in colour of the reagent. Any colour change taking place after two minutes should be disregarded. At the end of the test, rinse the comboplate® thoroughly with water as the purple colour may stain the wells. Stubborn stains can be removed by dissolving with a few drops of 5.5 M hydrochloric acid, and rubbing with a cotton bud or wooden skewer as previously described. The Iodoform Test for Methyl Ketones If the 2,4-DNP test indicates that an aldehyde or ketone is present, and if the Tollen’s and Schiff’s tests suggest that the compound is not an aldehyde, then the iodoform test can be used to distinguish methyl ketones from other ketones. Iodoform (CHI3) is produced when a methyl ketone reacts with iodine solution in the presence of aqueous sodium hydroxide. Other ketones do not form iodoform. The iodoform reaction must be performed after: i. the 2,4-DNP test to ensure that alcohols are excluded, since secondary alcohols will also give a positive result, and ii. the Tollen’s or Schiff’s tests since acetaldehyde will give a positive result. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Avoid dropping any of the organic reagents onto the skin. If any of these chemicals is spilled onto the skin, wash the affected area with copious amounts of water. Do not allow any flames to be brought near the organic reagents used in this experiment. They are all flammable. Do not inhale the vapours of the alcohol, ester, aldehyde, ketone and carboxylic acid as they are poisonous and may cause respiratory damage. Silver nitrate solution is poisonous and can cause heavy metal poisoning. Take care when using this solution. Silver is also an expensive metal, therefore the solution must not be wasted! The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 20 The mixture of the silver nitrate, sodium hydroxide and ammonia solutions (“Tollen’s reagent”) should never be heated. The solution formed from the reaction of an aldehyde with Tollen’s reagent should also never be heated, as it may become explosive. The acetic, nitric and hydrochloric acids recommended for cleaning are corrosive. If any acid is spilt on the skin, the affected area must immediately be rinsed with copious amounts of water. Severe burns must receive medical attention. Never point a propette or a syringe containing acid upwards. A momentary lapse of concentration can result in a nasty accident. If any acid is squirted into the eye, immediately rinse the eye out under running water. Always have a dilute solution of sodium hydrogencarbonate (household baking soda), or milk close by to apply to the injury. These substances will help neutralise the acid in the eye. The patient should be referred to a doctor. Dispose of all organic waste in the waste container provided, and not down the drain! MODEL ANSWERS TO QUESTIONS Part 1: The 2,4-Dinitrophenylhydrazine Test for Aldehydes and Ketones Q1. What do you observe immediately and after 5 minutes? Prepare a table like the one below. A1. TABLE 2: TESTING OF VARIOUS COMPOUNDS WITH 2,4-DNP REAGENT IMMEDIATE REACTION WITH 2,4 DNP REAGENT REACTION AFTER STANDING (~ 5 MINUTES) none none Formaldehyde (aqueous) Precipitate of bright, orange-yellow crystals forms. A greater volume of precipitate has deposited in the w ell. Propanone Colour of solution changes from yellow to orange. A precipitate of fine, needle-like orange crystals has formed. Formic acid None, or the solution may become pale orange in colour. The colour fades w ith standing. N one Ethyl acetate N one N one *Butyraldehyde Colour changes slightly to pale orange but may not be very evident. A yellow precipitate forms w ith stirring COMPOUND Methanol * The results for butyraldehyde as an alternative aldehyde are also given. Q2. Name the class to which each of the compounds used in this experiment belongs (eg. alcohol, alkane, ester, etc.) A2. methanol alcohol formic acid carboxylic acid aldehyde ethyl acetate ester formaldehyde ketone *butyraldehyde aldehyde propanone The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 21 Q3. Use the results in Table 2 and your answer to question 1 to explain how the 2,4-DNP test helps to distinguish aldehydes and ketones from other -C-O- and -C=O containing compounds. A3. Aldehydes and ketones form yellow, orange or red precipitates when they react with 2,4-dinitrophenylhydrazine reagent. Other carbonyl containing compounds like esters and carboxylic acids do not form precipitates with 2,4-DNP. Compounds containing the -C-O- group, such as alcohols, also do not react with the 2,4-DNP. Part 2: Tests to Distinguish Between Aldehydes and Ketones 2.1: Reduction of Ammoniacal Silver Nitrate Solution - Tollen’s Test Q1. What do you observe after 5 to 10 minutes? Prepare a table like the one below. A1. TABLE 3: TESTING OF ALDEHYDES AND KETONES WITH AMMONIACAL SILVER NITRATE SOLUTION NAME OF ALDEHYDE/KETONE REACTION WITH AMMONIACAL SILVER NITRATE SOLUTION Formaldehyde (aqueous) The solution blackens as soon as formaldehyde is added. A very shiny precipitate of silver forms and deposits on the bottom of the w ell. After 5 minutes, the entire bottom and sides of the w ell are covered w ith a silver mirror. Propanone No reaction. There is no silver mirror. *Butyraldehyde The solution blackens after a few seconds and a silver precipitate is detected after a short w hile. The silver also deposits on the w alls and bottom of the w ell, but is slightly darker and not as shiny as the silver mirror formed w ith formaldehyde. Q2. How can Tollen’s test be used to differentiate between an aldehyde and a ketone? A2. Aldehydes react with the ammoniacal silver nitrate solution to form a silver mirror. Ketones do not react and therefore a silver mirror cannot be detected. Q3. The reaction which occurs when an aldehyde is added to Tollen’s reagent is: RCHO(l) + 2[Ag(NH3)2]OH(aq) → RCOONH4(aq) + 2Ag(s) + 3NH3(aq) + H2O(l) i. ii. What type of reaction is this? Explain your answer to i above. A3. i. ii. It is a redox reaction. The silver ion in the [Ag(NH3)2]OH molecule has an oxidation number of +1. The silver atom in the silver precipitate has an oxidation number of 0. The silver ion has gained an electron and has therefore been reduced. The carbon atom in the aldehyde molecule (RCHO) has an oxidation number of 0. The carbon atom in the RCOONH4 molecule has an oxidation number of +2. The carbon atom has lost electrons and has therefore been oxidised. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 22 Part 2: Tests to Distinguish between Aldehydes and Ketones 2.2: Schiff’s Test to Distinguish between Aldehydes and Ketones Q1. What do you observe in each well? Prepare a table like the one below. A1. TABLE 4: TESTING OF ALDEHYDES AND KETONES WITH SCHIFF’S REAGENT ALDEHYDE/KETONE TESTED REACTION WITH SCHIFF'S REAGENT Formaldehyde (aqueous) The solution changes immediately to a dark violet colour. Stirring is not usually necessary. Propanone The solution remains colourless. No reaction takes place. *Butyraldehyde Within a few seconds of stirring, the solution changes to violet in colour. The colour becomes more intense w ith time. Q2. Based upon the results in Table 4 above, explain how the Schiff’s test allows one to distinguish between aldehydes and ketones. A2. Aldehydes react with Schiff’s reagent and form a violet or purple coloured product in solution. Ketones do not react, therefore a colourless solution remains. Q3. Examine the structures of formaldehyde and propanone below. i. ii. When formaldehyde reacts with Tollen’s reagent, what is it converted to? Write equations to illustrate your answer. Explain why propanone cannot react in the same way. A3. i. Formaldehyde is converted to formic acid, which in the presence of ammonia gives ammonium formate. CH2O(aq) + 2[Ag(NH3)2]OH(aq) → HCOONH4(aq) + 2Ag(s) + 3NH3(aq) + H2O(l) ii. The structure of propanone shows that it does not have a hydrogen atom bonded to the carbonyl carbon atom. It is therefore not oxidised by Tollen’s reagent. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 23 Q4. The figure below shows a scheme for testing substances to check whether they are aldehydes or ketones. Complete the chart by filling in the missing words related to the experiments completed in this worksheet. A4. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 24 FUNCTIONAL GROUP ANALYSIS The Iodoform Reaction TEACHER'S GUIDE AND MODEL ANSWERS Introduction Some ketones have a methyl group bonded to the carbonyl carbon atom. These are called methyl ketones. Other ketones, called non-methyl ketones, do not have a methyl group adjacent to the carbonyl group. It is possible to distinguish between these two kinds of ketones, by observing whether iodoform (CHI3(s)) is formed when a ketone is mixed with iodine and sodium hydroxide solutions. In this experiment learners will react various ketones, an aldehyde and some alcohols with iodine and sodium hydroxide solutions. The results of these reactions will help them determine which of the ketones is a methyl ketone and which is not. It will also show that other compounds can be oxidised to methyl ketones, and hence form iodoform. 1. Chemicals All the chemicals required are found in the requirements table preceding the experiment. If the ketones and alcohols listed are not available, then other ketones and alcohols can be used. Remember to provide a methyl ketone and a non-methyl ketone so that it can be shown that the iodoform test distinguishes between the two. It is a good idea to also test a number of primary, secondary and tertiary alcohols. The potassium iodide-iodine reagent (sometimes referred to as iodine solution) should be kept in a cool dark place because it is decomposed by heat and light. If the teacher would like the students to test an unknown substance, then the compound provided should not be a quinone or hydroquinone as these also give a positive result in the iodoform test. If the compound to be tested is insoluble in water, it may be necessary to dissolve it in a little dioxan. However, the dioxan may be contaminated with impurities that give a positive iodoform reaction, so it is necessary to subject the dioxan alone to the iodoform test. 2. Apparatus The propettes and microspatulas are components of the RADMASTE Microchemistry kits. The organic comboplate is manufactured from a material which is resistant to organic solvents and must be used in this experiment, because the conventional comboplate will be dissolved by the ketones, aldehyde and some of the alcohols used. Gloves should be worn throughout the experiment to protect the skin from the corrosive nature of the chemicals used. The teacher must carry out a risk assessment to decide whether protective glasses should be worn by the students. The teacher must also ensure that a waste container is provided into which all organic waste can be disposed. 3. Hints Since most of the chemicals used in the experiment are colourless, it is a good idea to advise students to label their propettes prior to the experiment to prevent these from becoming muddled. Two or three students can share propettes of the ketones, aldehyde and alcohols because only a few drops of each is required. Since there are more than six compounds to be tested, students should be encouraged to work in pairs. One student can test some of the compounds, while the other tests the remainder. The results can be shared. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 25 Propanone has the tendency to flow from the propette tip, especially when the temperature of the surroundings is relatively high. The propette containing the propanone should be handled carefully and very little pressure applied to the bulb as drops of the liquid may fall freely into the well. Do not add an excess of the propanone or other compounds to be tested because the iodoform reaction is only successful when the iodine and sodium hydroxide solutions are added in excess. The ketones have been chosen in such a way that one ketone is a methyl ketone (propanone) and the other is not (cyclohexanone). Only the former gives a positive iodoform reaction. The formaldehyde is representative of the majority of aldehydes (except acetaldehyde) which do not react with hypoiodite solution. The alcohols have been selected in such a way to show that only certain alcohols give a positive iodoform reaction, as explained earlier. Propanone reacts immediately with the hypoiodite solution to produce iodoform as a yellow suspension. This gives the solution a milky yellow/cloudy appearance. The ethanol and secondary butyl alcohol do not react as quickly as the propanone. For this reason, a waiting period of 2 to 3 minutes is allowed for in the experiment, after which time these solutions also appear milky yellow. The cyclohexanone is slightly soluble in cold water, therefore when the sodium hydroxide and iodine solutions are added, two layers may be seen. After addition of the iodine solution the upper organic layer may retain the brown colour of iodine but this fades quickly, and it is obvious that no iodoform has been obtained with cyclohexanone. Iodoform crystals will not have settled at the bottom of the relevant wells after the 2 minute standing period. The procedure therefore advises that the comboplate be left to stand for another 5 to 10 minutes, during which time the iodoform precipitates at the bottom of each well in which a positive result is obtained. The longer the waiting period, the greater the volume of iodoform that will precipitate and the easier the solid will be detected at the bottom of a particular well. Individual crystals of iodoform cannot be seen but rather a fine, pale yellow precipitate settles at the bottom of a well. Sometimes a solid is difficult to detect because the entire solution is still cloudy. There are two ways in which it can be proved that there is a precipitate in the well: the bottom of the well can be scratched with the narrow end of a microspatula and/or the liquid contents in the comboplate can be discarded as waste. With the former method, scratch marks can be seen in the precipitate at the bottom of the well proving that a solid is present. When the latter method is used, the precipitates adhere to the bottom of the relevant wells and can easily be seen. Those wells in which a negative result is obtained will of course be empty when the solution is discarded. The precipitates can be removed from the comboplate by first rinsing with water and then using a cotton bud - or the pointed end of a wooden skewer around which a small strand of cotton wool has been wrapped - to remove any solid residue. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Propanone, in particular, has a low surface tension and drops of the liquid may fall from the tip of the propette without pressure being placed on the bulb. Avoid dropping any of the organic reagents onto the skin. If any of these chemicals is spilled onto the skin, wash the affected area with copious amounts of water. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 26 Do not allow any flames to be brought near the organic reagents used in this experiment. They are all flammable. Do not inhale the vapours of the ketones, aldehyde and alcohols as they are poisonous and may cause respiratory damage. Keep the bottles of all organic liquids closed so as to minimise vaporisation. The halogen solutions are corrosive. Do not drop the iodine solution onto skin or fabrics. If contact is made with the skin, wash the affected area with copious amounts of water. Keep the bottle containing the iodine solution in a cool, dark place as it decomposes in the presence of light and heat. Never point a propette or a syringe containing acid or base upwards. A momentary lapse of concentration can result in a nasty accident. If any base is squirted into the eye, immediately rinse the eye out under running water. Always have a dilute solution of boric acid close by to apply to the injury. This will help neutralise the base in the eye. The patient should be referred to a doctor. Dispose of all organic waste in the waste container provided, and not down the drain! MODEL ANSWERS TO QUESTIONS Q1. What do you observe after 2-3 and after 5-10 minutes? Prepare a table like the one below. A1. TABLE 1: TESTING OF VARIOUS COMPOUNDS WITH HYPOIODITE (I2/KI - NaOH) SOLUTION COMPOUND APPEARANCE OF WELL CONTENTS AFTER 2 TO 3 MINUTES PRECIPITATE (CHI3(s)) FORMATION? Propanone A milky yellow turbidity is present. Yes Cyclohexanone Tw o layers may be seen. The low er layer is colourless w hile the upper layer has a faint iodine (brow n) colour. This later fades. No Formaldehyde (aqueous) The solution is colourless and clear. No Methanol A clear, colourless solution remains. No Ethanol Appears cloudy (milky yellow ). Yes Secondary butyl alcohol A milky yellow turbidity is present. Yes Tertiary butyl alcohol A colourless, clear solution remains. No *Propan-1-ol A colourless, clear solution remains. No Q2. What do you notice in the wells where turbidity was observed earlier? A2. A pale, yellow solid has settled at the bottom of those wells in which the contents were cloudy (i.e those wells which contained propanone, ethanol and secondary butyl alcohol). Q3a. What do you see in step 7 of the procedure? A3a. Scratch marks are evident in the pale yellow solid at the bottom of the wells in which propanone, ethanol and secondary butyl alcohol were tested. This proves that a precipitate has formed with these compounds. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 27 Q3b. What do you notice in step 8 of the procedure? A3b. There is a pale yellow precipitate at the bottom of the wells where propanone, ethanol and secondary butyl alcohol were tested. Q4. Describe the odour. A4. The solid has a characteristic odour similar to that of some antiseptic substances. Q5. Examine the structures of propanone and cyclohexanone alongside. What is the difference in structure between these two ketones, besides the fact that one ketone is cyclic and the other aliphatic? A5. Propanone is a methyl ketone i.e its molecules contain a methyl group bonded to the carbonyl carbon atom. Cyclohexanone is not a methyl ketone. Q6. Based upon your answer to question 1 and the observations made in this experiment, how does the iodoform test help to distinguish methyl ketones from non-methyl ketones? A6. Methyl ketones react with hypoiodite solution to form iodoform, seen as turbidity or a precipitate of pale yellow crystals. Non-methyl ketones do not form iodoform. Q7. The reaction of a methyl ketone with iodine solution in the presence of aqueous sodium hydroxide is shown in the equation below: RCOCH3(l) + 3I2(aq) + 4NaOH(aq) → RCOONa(aq) + CHI3(s) + 3NaI(aq) + 3H2O(l) where R = H, alkyl or aryl group i. ii. A7. Which part of the ketone molecule has been involved in the formation of an iodoform molecule? Use your answer to i to explain why cyclohexanone gives a negative reaction in the iodoform test. i. The methyl group attached to the carbonyl carbon atom in the ketone molecule is involved in the reaction which produces iodoform molecules. ii. Cyclohexanone does not contain a methyl group attached to the carbonyl carbon atom, and therefore iodoform cannot be produced. (Reaction with iodine does occur but the product is not iodoform.) Q8. Formaldehyde gives a negative result in the iodoform test. Do you think that the iodoform test could be used to distinguish methyl ketones from all aldehydes? Explain your answer with reference to the equation given in question 3. A8. No. The equation shows that a positive iodoform test will be obtained with a compound having the structure RCOCH3 where R is an H atom, alkyl or aryl group. The compound HCOCH3 is an aldehyde (acetaldehyde). Acetaldehyde will also produce iodoform with hypoiodite solution because, like methyl ketones, its molecules possess a methyl group bonded to the carbonyl carbon atom: HCOCH3(l) + 3I2(aq) + 4NaOH(aq) → HCOONa(aq) + CHI3(s) + 3NaI(aq) + 3H2O(l) The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 28 Q9. The equation for the initial reaction between an alcohol (primary or secondary) and iodine solution in the presence of aqueous sodium hydroxide is: RCH(OH)R’(l) + I2(aq) + 2NaOH(aq) → RCOR’(aq) + 2NaI(aq) + 2H2O(l) where R and R’ = H, alkyl or aryl group i. ii. iii. A9. According to the equation, which alcohols should react with the hypoiodite solution? Use the equation to explain why certain alcohols are able to give a positive iodoform reaction. Why does ethanol give a positive iodoform test but methanol does not? (Hint: look at the reaction equation). i. Alcohols whose molecules have the CH3-C-OH structure. (Let the students write down the structures of various kinds of alcohols that have the formula RCH(OH)R’. This will make it easier to identify those alcohols which do not have a methyl group adjacent to the OH-bonded carbon atom, and which will therefore not produce a methyl ketone.) ii. The equation shows that under the conditions of the reaction, certain alcohols are oxidised to methyl ketones. We know that such ketones react further with the hypoiodite solution to produce iodoform. iii. Alcohols having the structure RCH(OH)CH3 will give a positive result, where R is an H atom, alkyl or aryl group. The compound HCH(OH)CH3 is ethanol. The ethanol is oxidised to acetaldehyde which, as discussed previously, reacts with hypoiodite solution to produce iodoform. Methanol has the structure CH3OH. There is no other methyl group attached to the OH-bearing carbon atom, therefore a negative result is obtained with methanol. (A methanol molecule has a methyl group, but it is bonded directly to the OH.) Q10. Refer back to Table 1. Write down the reactions taking place for those ketones and alcohols which gave a positive iodoform test. A10. Propanone Ethanol sec-Butyl alcohol CH3COCH3 + 3I2 + 4NaOH → CH3COONa + CHI3 + 3NaI + 3H2O a) CH3CH2OH + I2 + 2NaOH → HCOCH3 + 2NaI + 2H2O b) HCOCH3 + 3I2 + 4NaOH → HCOONa + CHI3 + 3NaI + 3H2O a) CH3CH2CH(OH)CH3 + I2 + 2NaOH → CH3CH2COCH3 + 2NaI + 2H2O b) CH3CH2COCH3 + 3I2 + 4NaOH → CH3CH2COONa + CHI3 + 3NaI + 3H2O Q11. You are working in a company laboratory. You have been asked to use 1-propanol in a particular experiment. You find a cupboard in which there is a shelf for alcohols, but the bottles are not labelled. You know that ethanol and 1-propanol are used often. Explain how you will use the iodoform test to locate a bottle of 1-propanol in the presence of bottles of ethanol. Predict the outcome of your investigation and refer to the structures of the alcohol compounds when necessary. A11. The student should recognise that ethanol gives a positive iodoform test. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 29 FUNCTIONAL GROUP ANALYSIS Tests for Identifying and Classifying Alcohols TEACHER'S GUIDE AND MODEL ANSWERS Introduction Both alcohols (R-O-H) and ethers (R-O-R’) are neutral, oxygen containing compounds. The purpose of the sodium test (Part 1) is to enable one to distinguish between alcohols and ethers by observing how each compound reacts when sodium metal is added to it. The two tests in Part 2 allow one to distinguish primary, secondary and tertiary alcohols from one another, by observing the reactivity each class of alcohol displays. Secondary and tertiary alcohols both form turbid mixtures with Lucas’ Reagent. When an unknown alcohol is tested and forms a turbid mixture, the chemist may not be able to determine whether the alcohol is secondary or tertiary in structure. To determine conclusively which of the alcohols is the secondary and which is the tertiary structure, the chemist needs to mix each of the alcohols with concentrated hydrochloric acid. Chromic Anhydride Reagent, also called Jones' Reagent, is a solution of chromic anhydride (chromium trioxide - CrO3) in dilute sulphuric acid. It rapidly oxidises primary and secondary alcohols. Tertiary alcohols do not react visibly in less than two minutes under the test conditions. Primary alcohols are oxidised to carboxylic acids, and secondary alcohols form ketones: 3RCH2OH(l) + 4CrO3(aq) + 6H2SO4(aq) → 3RCOOH(l) + 2Cr2(SO4)3(s) + 9H2O(l) 3RR'CHOH(l) + 2CrO3(aq) + 3H2SO4(aq) → 3RR'CO(l) + Cr2(SO4)3(s) + 6H2O(l) The chromium sulphate (Cr2(SO4)3(s)) is a greenish-blue precipitate. As it is formed, it gives the test solution a blue-green, opaque appearance. The precipitate later settles and the test solution becomes clear. 1. Chemicals All the chemicals required are listed in the worksheet. Ice is required as well as boiling water - at least enough to fill every student's lunch box or to fill a communal container eg. a bucket of some sort (see step 3). Instead of ice, students can use cold water to cool the reaction mixture. This can be tap water (depending on the ambient temperature) or cold water from a fridge. 2. Apparatus The vials, propettes, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the comboplate used with aqueous solutions can be affected by the organic reagents. A kettle (or a stove with a pot) may be required to boil the water in Part 2.1. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 30 3. Hints Since the organic reagents used in all parts of the experiment are colourless, it is a good idea to advise students to label their propettes prior to the experiment to prevent these from becoming muddled. Two or three students can share propettes of the alcohols and Lucas Reagent in Part 2.1, as well as the acetone and Chromic Anhydride Reagent in Part 2.2 because only a few drops of each is required. Part 1: The Sodium Test to Distinguish Between Alcohols and Ethers The aim in Part 1 is to show that the reaction between an alcohol and metallic sodium can be used to distinguish alcohols from ethers, which do not react with sodium. Since the presence of water in samples of the ethanol and ether is unavoidable, Part 1 focuses mainly on the differences in the rate of hydrogen production when sodium is added to ethanol and ether. The reaction of sodium with ethanol is vigorous (shown by the fizzing sound and the movement or ‘darting’ of the sodium across the surface of the ethanol) and rapid (hydrogen bubbles are given off very quickly). The sodium is used up in the reaction after a short while. In contrast, when sodium is added to the ether the reaction is slow without any fizzing or rapid movement of the piece of sodium. The sodium reacts with water which was not removed during the drying of ether with magnesium sulphate. Since the water is present only as a trace impurity in the ether, the bubbles of hydrogen are given off more slowly with time as the concentration of water molecules decreases. After about 10 minutes, the reaction has ceased and the size of the piece of sodium is not much smaller than when first added to the ether. Coarse granules of a white solid are evident at the bottom of the well. This is sodium hydroxide which has precipitated in the ether during the reaction of sodium with water: 2H2O(l) + 2Na(s) → 2NaOH(s) + H2(g) Students must be made aware that ether escapes very readily from the propette, which must therefore not be pointed upwards. Most of the water may usually be removed with anhydrous magnesium or calcium sulphate, or alternatives. At the drying step in Part 1 students should be advised to keep the container of magnesium sulphate closed at all times, otherwise the solid will absorb moisture from the atmosphere (therefore rendering it useless as a drying agent). The unreacted sodium in the well containing the ether must be destroyed by adding a little cold ethanol or methanol. Once all the sodium has reacted, the remaining solution can be washed down the sink. Part 2.1: Lucas’ Test to Distinguish Between 10, 20 and 30 Alcohols If the room temperature is below 250C, students should be advised to keep the propettes containing tertiary butyl alcohol in a little warm water. Tertiary butyl alcohol freezes in the temperature range 24 - 25.50C, and if not kept at a temperature above freezing it will form crystals that block the stem and bulb of the propette. The temperature of the water must be maintained at 27 to 280C by mixing different volumes of hot and cold water as required. It is suggested that the water temperature be raised to 280C before the comboplate is placed in the bath. During the 5 minute reaction period, the temperature should decrease by one degree only to 270C. This will prevent the need to add hot water to the bath while the comboplate is being warmed. The volume of water used in the water bath should be kept low, and the comboplate must not be pushed into the water as it may become immersed in the bath. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 31 When Lucas’ Reagent is added to the alcohols listed n-butyl alcohol does not react, secondary butyl alcohol does not react immediately but will form the alkyl chloride after warming for about 5 minutes at 27 to 280C, and tertiary butyl alcohol reacts almost immediately to produce a turbid solution which becomes more turbid while the comboplate is on the water bath. In order for students to be aware of the different reaction rates, the test solutions are observed immediately after adding Lucas’ Reagent to the alcohols, as well as after being kept at 27 to 280C. The conclusion can then be made that primary alcohols do not react with Lucas’ Reagent, secondary alcohols react slowly with heating and tertiary alcohols react quickly even in the cold. The colour of the turbid solutions produced after incubation is almost the same as the colour of the opaque plastic of the Organic comboplate. This may make it difficult for students to observe the turbidity in the well to which the secondary butyl alcohol was added, since the volume of alkyl chloride produced with the secondary alcohol is small (much less than that for tertiary butyl alcohol). In this case, students should be advised to hold the comboplate up to the light and observe the wells from both above and the underside of the comboplate. Lucas’ Test has an extension which allows one to distinguish between secondary and tertiary alcohols after the initial reaction with the hydrochloric acid-zinc chloride reagent has been used to exclude primary alcohols. In Part 2.1, the procedure requires that both secondary and tertiary butyl alcohol are mixed with 11 M hydrochloric acid. The secondary alcohol does not react and the solution remains clear. The tertiary butyl alcohol reacts within 15 seconds to produce a suspension of tertiary butyl chloride, which gives the solution a milky, white appearance: Since the volumes of alcohols and Lucas’ Reagent used are small, the waste solutions may be decanted onto a thick pile of paper towel. The organic residue evaporates quicky in the open air and the towel can then be thrown away. If this is not a possible option, then the chlorinated organic waste much be discarded into a specially designated waste container to ensure that it is not mixed with non-chlorinated waste. Part 2.2: The Chromic Anhydride Test to Distinguish 10 and 20 Alcohols from 30 Alcohols The oxidation of n-butyl alcohol and secondary butyl alcohol with Chromic Anhydride Reagent is instantaneous. The reactions are accompanied by vigorous bubbling, after which an opaque bluegreen coloured solution is produced. The solution formed with the secondary butyl alcohol also has a yellow tinge. The opaque solutions are visible within 3 to 5 seconds of adding the Chromic Anhydride Reagent to the alcohols, after which the solution becomes clear and a dark blue precipitate settles at the bottom of each well. It is this precipitate which actually produces the initial opaque blue-green solution, as the solid does not settle until later. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 32 Tertiary butyl alcohol does not react and there is no change in the deep orange colour of the Chromic Anhydride Reagent. Students need to be careful when adding the reagent to the primary and secondary alcohols, as the bubbling is so vigorous that the solutions may spill out of the wells. The comboplate should not be lifted for observation until after the reaction has stopped. The Chromic Anhydride Reagent is a strong oxidant and may oxidise aldehydes, enols, phenols and other substances that occur as impurities in the alcohol samples. These reactions are slower than the oxidation of the alcohols, and a time limit of 5 seconds has been set in which observations can be made. Any other oxidations taking place after 5 seconds are to be disregarded. The precipitation of the blue-green chromium sulphate solid, however, takes place after 5 seconds. Students should be made aware that the settling of the precipitate is not due to a chemical reaction, but is a change in the appearance of the solutions following the initial oxidation reactions. The changes should still be noted shortly after the oxidations of the primary and secondary alcohols have occurred, usually within 30 seconds. As in Part 2.1, the volumes of organic waste produced are small and can be decanted onto a thick pile of paper towel to allow evaporation to take place in the open air. Alternatively, the waste can be discarded into a container before rinsing the comboplate with water. The Iodoform Test for Methyl Ketones If it is suspected that an alcohol may be secondary or tertiary in structure, then the iodoform test may also be carried out on the alcohol provided that other tests have been done to exclude the presence of an aldehyde and ketone. The molecules of secondary alcohols may contain a methyl group adjacent to the -OH bearing carbon atom. Under the conditions of the iodoform test the secondary alcohol is oxidised to a methyl ketone, which in turn produces iodoform - CHI3. (See Chapter 1: Experiment 3 for details.) Tertiary alcohols do not react with the sodium hypoiodite solution and iodoform is not produced. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Diethyl ether is a special example. Avoid dropping any of the organic reagents onto the skin. If any of these chemicals is spilled onto the skin, wash the affected area with copious amounts of water. Avoid dropping the Lucas’ and Chromic Anhydride Reagents onto the skin as they are highly corrosive. (Lucas’ Reagent is prepared with concentrated hydrochloric acid, while the Chromium Anhydride Reagent contains sulphuric acid.) If spillage occurs, rinse the affected area with copious amounts of water. Do not allow any flames to be brought near the organic reagents used in this experiment. They are all flammable. Do not attempt to test for hydrogen gas (Part 1) using a flame as the ethanol and comboplate will catch alight. Do not inhale the vapours of the alcohols, acetone and ether as they are poisonous and may cause respiratory damage. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 33 11 M hydrochloric acid is extremely corrosive. Take care when handling the acid. Never point a propette or a syringe containing acid upwards. A momentary lapse of concentration can result in a nasty accident. If any acid is squirted into the eye, immediately rinse the eye out under running water. Always have a dilute solution of sodium hydrogencarbonate (household baking soda), or milk close by to apply to the injury. These substances will help neutralise the acid in the eye. The patient should be referred to a doctor. Do not dispose of any unreacted sodium in the sink. Ensure that all the sodium has reacted with cold ethanol before rinsing the comboplate. Dispose of all other organic waste in the waste containers provided, and not down the drain! MODEL ANSWERS Part 1: The Sodium Test: Using Metallic Sodium to Distinguish Alcohols from Ethers Q1. Prepare a table like the one below and record your observations. A1. TABLE 1: TESTING OF ALCOHOL AND ETHER WITH METALLIC SODIUM COMPOUND OBSERVATIONS Ethanol The sodium reacts rapidly w ith the alcohol, as show n by small bubbles quickly being given off. The piece of sodium "darts" around the w ell on the surface of the ethanol and a fiz z ing sound is heard. After about 3 to 5 minutes, all of the sodium has reacted. Diethyl Ether The piece of sodium sinks to the bottom of the w ell and small bubbles can be seen rising to the surface of the ether. There is no fiz z ing sound as the rate of reaction is slow er than for ethanol. The reaction slow s dow n considerably w ith time until only a few bubbles are given off at intervals. After about 8 to 10 minutes, the reaction has stopped. The piece of sodium has not decreased much in siz e and there are granules of a w hite solid at the bottom of the w ell. (Note that if the teacher has obtained a fresh, pure sample of ether w hich has not been contaminated w ith w ater, there should not be any reaction w ith sodium .) Q2. How can one distinguish between an alcohol and an ether using the sodium test? A2. Sodium reacts rapidly and vigorously with an alcohol. Hydrogen gas is formed and the sodium is used up in the reaction. Sodium reacts sluggishly with (the water in) an ether. Very little hydrogen gas is generated. The sodium is not all used up in the reaction and a precipitate is produced. Q3. Write down the equation for the reaction of ethanol with sodium. A3. 2CH3CH2OH(l) + 2Na(s) → 2CH3CH2ONa(l) + H2(g) Q4. Write down the reaction of water with sodium metal. Q4. 2H2O(l) + 2Na(s) → 2NaOH(aq) + H2(g) The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 34 Q5. The reaction of anhydrous magnesium sulphate with water is as follows: MgSO4(s) + 7H2O(l) → MgSO4.7H2O(s) i. ii. What is the function of the anhydrous magnesium sulphate in the sodium test? Why is it important to add anhydrous magnesium sulphate to the alcohol and ether prior to reaction with sodium metal? (Hint: look at your answer to question 1 above.) iii. Ether does not react with water. Explain why you observed a reaction when sodium was added to the diethyl ether. (Hint: look at your answer to question 1 above.) iv. Use your answer to iii. to explain why there was a difference in the rates of reaction when sodium was added to ethanol and diethyl ether. v. What is the name of the precipitate that settled at the bottom of the well containing the ether? A5. i. It is a dehydrating agent. (It removes water from the alcohol and ether by forming a hydrate.) ii. The alcohol and ether may contain water as an impurity. Since water reacts with sodium metal to produce hydrogen, it must be removed from the alcohol and ether so that the results are interpreted correctly. iii. Water was present as an impurity in the diethyl ether and was not all removed with the magnesium sulphate. The small concentration of water molecules in the ether (and not the ether) reacted with the sodium metal. iv. A large concentration of ethanol molecules reacted with all of the sodium metal atoms, causing the rapid evolution of hydrogen gas. In contrast, there was only a small concentration of water molecules in the ether. The reaction of water with the sodium was therefore slower than that with ethanol. All the water molecules were used up before all the sodium atoms reacted, resulting in cessation of hydrogen production. v. Sodium hydroxide (not soluble in ether). Part 2.1 Lucas’ Test for Differentiating Between Primary, Secondary and Tertiary Alcohols Q1. Prepare a table like the one below and record your results. A1. TABLE 2: REACTION OF PRIMARY, SECONDARY AND TERTIARY ALCOHOLS WITH LUCAS’ REAGENT ALC OH OL IMMED IATE R EAC TION WITH LU C AS' R EAGEN T OB SER VATION S AFTER 5 MIN U TES AT 27 TO 280C n-butyl alcohol N o reaction. The solution remains clear. N o reaction. The solution is still clear. secondary butyl alcohol N o reaction. The solution is clear. A (slow ) reaction has occurred, as show n by a slight turbidity in the w ell. terti ary butyl alcohol A reaction occurs w ithin a few seconds of adding Lucas' R eagent and the w ell contents become turbid. The reaction w hich started at room temperature has continued because the turbidity in the w ell has increased. *ethanol N o reaction. Solution is clear. N o reaction. Solution is still clear. * The results for ethanol as an alternative primary alcohol have also been provided. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 35 Q2. Prepare a table like the one below and record your observations. A2. TABLE 3: REACTION OF SECONDARY AND TERTIARY ALCOHOLS WITH CONCENTRATED HYDROCHLORIC ACID ALC OH OL OB SER VATION S secondary butyl alcohol N o reaction. The solution remains clear. terti ary butyl alcohol A reaction occurs w ithin 10 to 15 seconds to produce a milky, w hite mixture in the w ell. Q3. Classify the alcohols used in this experiment as primary, secondary or tertiary. A3. n-butyl alcohol primary secondary butyl alcohol secondary tertiary butyl alcohol tertiary *ethanol primary (Note: if students find it difficult to classify certain alcohols as primary, secondary or tertiary, let them draw the structures of each alcohol so that they can see the number of carbon substituents bonded to the OH-bearing carbon atom.) Q4. Use your answer to question 1 and the results in Table 2 to explain how mixing an alcohol of unknown structure with Lucas’ Reagent can help one to determine whether it is primary, secondary or tertiary. A4. If an alcohol of unknown structure is mixed with Lucas’ Reagent and the solution remains clear after 5 minutes at 27 - 280 C, the alcohol is primary in structure. If the solution becomes turbid after 5 minutes at 27 to 280 C, the alcohol is secondary in structure. If the solution becomes turbid immediately (i.e in the cold), the alcohol is tertiary in structure. Q5. Write down an equation for the reaction of tertiary butyl alcohol with concentrated hydrochloric acid. A5. Q6. Explain why the solution became milky white in appearance after adding 11 M HCl(aq) to the tertiary butyl alcohol. A6. Tertiary butyl chloride formed as a result of the reaction between the alcohol and concentrated hydrochloric acid. The liquid droplets remained in suspension, making the solution appear milky white. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 36 Q7. A student is given two alcohols with which to perform Lucas’ Test. These are cyclohexanol and 2-methyl-2-propanol. The bottles containing each alcohol are labelled, but the propettes used are not. The student accidentally muddles the propettes. When drops of each alcohol are mixed with Lucas’ Reagent and kept at 270 C, both solutions become turbid. How can the student find out which alcohol has been placed in which propette? A7. Students should give answers similar to the following: The student should mix each alcohol with 11 M hydrochloric acid in the comboplate and observe the reaction mixtures. If the alcohol from a particular propette reacts with the HCll(aq) to form a cloudy solution almost immediately, then that propette contains the tertiary alcohol. If the alcohol from a particular propette shows no reaction with the acid (the solution remains clear in the comboplate), then that propette contains a secondary alcohol. Cyclohexanol is a secondary alcohol and did not react with HCll(aq). 2-methyl-2-propanol is tertiary butyl alcohol and formed a cloudy mixture with HCll(aq). Part 2.2: The Chromic Anhydride Test: Distinguishing Tertiary Alcohols from Primary and Secondary Alcohols Using Chromic Anhydride Reagent (Jones= Reagent) Q1. Prepare a table like the one below and record your observations. A1. TABLE 4: REACTION OF PRIMARY, SECONDARY AND TERTIARY ALCOHOLS WITH CHROMIC ANHYDRIDE REAGENT ALCOHOL REACTION WITHIN 3 TO 5 SECONDS OF ADDING CHROMIC ANHYDRIDE REAGENT CHANGE IN APPEARANCE OF WELL CONTENTS AFTER INITIAL REACTION n-butyl alcohol Reacts vigorously w ith bubbling w ithin about 4 seconds. The test solution becomes an opaque, green/blue colour. The solution loses turbidity (becomes clear w ith a blue tint). A dark blue/green precipitate settles at the bottom of the w ell. secondary butyl alcohol Reacts vigorously w ithin about 4 seconds. There is bubbling and an opaque yellow /green solution is formed. The solution becomes clear w ith a slight green tint. A dark blue/green precipitate settles at the bottom of the w ell. tertiary butyl alcohol No reaction. The solution remains deep orange in colour. No change. The solution is still deep orange in colour. *ethanol Reacts vigorously w ith bubbling w ithin the 3 to 5 second time span. An opaque blue/green solution results. The turbidity clears and a dark blue/green precipitate settles at the bottom of the w ell. * The results for ethanol as an alternative primary alcohol have been provided. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 37 Q2. How does the Chromic Anhydride Test distinguish primary and secondary alcohols from tertiary alcohols? A2. Primary and secondary alcohols react with the Chromic Anhydride Reagent within 5 seconds to form opaque, blue-green mixtures. Tertiary alcohols do not react and the deep orange colour of the Chromic Anhydride Reagent remains. Q3i. Write down an equation for the reaction of n-butyl alcohol with Chromic Anhydride Reagent. (The reagent = CrO3 + H2SO4) A3i. Q3ii. What type of compound is formed in the reaction between a primary alcohol and Chromic Anhydride Reagent? (Hint: use the answer to 3i. above.) A3ii. A carboxylic acid. Q4i. Write down an equation for the reaction of secondary butyl alcohol with Chromic Anhydride Reagent. A4i. Q4ii. What type of compound is formed in the reaction between a secondary alcohol and the Chromic Anhydride Reagent? (Hint: use the answer to A4i. above.) A4ii. A ketone. Q5. During the mixing of a primary or secondary alcohol with Chromic Anhydride Reagent, the following change occurs (equation unbalanced): CrO3(aq) + H2SO4(aq) → Cr2(SO4)3(s) Q5i. What kind of reaction has taken place? Explain by examining the partial reaction equation given. A5i. A redox reaction. The oxidation number of chromium has changed from +6 in the red CrO3 to +3 in the blue-green Cr2(SO4)3. Chromium has therefore gained electrons and has been reduced. (In each reaction, the primary or secondary alcohol is oxidised.) Q5ii. What is the blue-green precipitate that was observed at the bottom of each well in which a reaction occurred? A5ii. Chromium sulphate, Cr2(SO4)3(s) Q5iii. Why did the test solutions containing primary and secondary alcohols, initially become bluegreen in colour? A5iii. When the redox reaction occurred in each well, chromium sulphate was formed. It remained in suspension for a few seconds before precipitating. The solid particles made the test solutions appear blue-green in colour. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 38 Q6. Draw the chart below and complete it by filling in the missing words related to the experiments carried out in this worksheet. A6. k The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 39 FUNCTIONAL GROUP ANALYSIS Tests for Unsaturated Hydrocarbon Compounds TEACHER'S GUIDE AND MODEL ANSWERS Introduction Alkanes are hydrocarbon compounds whose molecules contain carbon-carbon single bonds. All have the general formula CnH2n+2 where n is any integer. They are often referred to as saturated hydrocarbons because their molecules contain the maximum possible number of hydrogen atoms per carbon atom, and because they consist only of carbon and hydrogen. Alkenes are hydrocarbon compounds whose molecules contain one or more carbon-carbon double bonds. They are often referred to as being unsaturated because the presence of a double bond means that they lack hydrogen atoms as compared with related alkanes. The general formula for alkenes is CnH2n. The molecules of alkyne compounds contain one or more carbon-carbon triple bonds. Like alkenes, they are often termed as unsaturated hydrocarbons. Tests to Distinguish Alkanes from Alkenes and Alkynes Alkanes are chemically inert to most reagents mainly because all the electrons in an alkane molecule are involved in carbon-carbon and carbon-hydrogen bonding. (Alkanes do however react with oxygen under appropriate conditions.) In contrast, alkenes and alkynes react with a number of other compounds. This is because the carbon-carbon double and triple bonds in alkene and alkyne molecules have greater electron density than a carbon-carbon single bond. These electron rich sites can donate a pair of electrons to an electrophile (an electron-poor or electron-seeking reagent). This difference in reactivity between saturated and unsaturated hydrocarbon compounds is used as the chemical basis for differentiating between the two. One test involves the Addition of Bromine to Alkenes and Alkynes. When a dilute solution of bromine in water (or carbon tetrachloride) is mixed with an alkane, no reaction occurs due to the inertness of saturated hydrocarbon compounds to bromine. The brown colour of the bromine remains. Mixing the same bromine solution with an alkene or alkyne results in the rapid disappearance of the brown colour of the bromine, until a colourless mixture is obtained. Another test to distinguish between alkenes and alkynes is based on their reaction with cold, dilute potassium permanganate solution - called Baeyer's Test. Potassium permanganate is an oxidant. When mixed with an alkene, it oxidises the double bond in alkene molecules to yield 1,2- diols i.e. an -OH group is added to each of the two alkene carbon atoms. Such compounds are also called glycols. Alkynes are also oxidised by potassium permanganate to yield carboxylic acids. The purple permanganate (MnO4-) ion is reduced to brown manganese(IV) oxide, as seen by the change in colour of the solution from purple to brown when mixed with an alkene or alkyne. 1. Chemicals All the chemicals required are found in the requirements table preceding Parts 1 and 2 of the experiment. The aqueous bromine solution (commonly called bromine water) has a high vapour pressure and should be stored in a cool place to minimize loss of bromine vapour, which will cause a decrease in the concentration of bromine molecules in solution. The solution is also decomposed in the presence of light and should be kept in an amber bottle in a dark place. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 40 A solution of bromine in carbon tetrachloride may be used as a substitute for the aqueous bromine solution. However, due to its toxicity, the organic solution will no longer be readily available from chemical suppliers and will be very expensive to obtain commercially. Bromine is more soluble in carbon tetrachloride than in water and therefore the carbon tetrachloride solution does not lose bromine as readily as the aqueous solution. It also deteriorates with heat and light and should be stored as explained for the aqueous solution. The 0.1% potassium permanganate solution can be prepared by dissolving 0.100 g of solid potassium permanganate in a little water in a 100 ml volumetric flask, and then making the solution up to 100 ml. It may be better to prepare a stock solution of 1% potassium permanganate and then dilute this to 0.1% as required. Potassium permanganate solutions are decomposed by heat and light. The decomposition reaction produces manganese dioxide which catalyses further decomposition of the permanganate. For this reason the solutions must be protected adequately from heat and light, and should be stored in clean containers because dust particles can also promote degradation of the solution. A 0.1% solution of potassium permanganate in acetone can be used in place of the aqueous solution, but the acetone based solutions deteriorate rapidly. This option may be considered if the entire solution is used on the day of experiment. If the teacher would like the students to test unknown substances, then other saturated (alkanes) and unsaturated (alkenes and alkynes) hydrocarbon compounds may be used. It should be remembered that other classes of organic compounds may also rapidly decolourise both the bromine and permanganate solutions. 2. Apparatus The propettes and microspatulas are components of the RADMASTE Microchemistry kits. An ordinary comboplate may be used in this experiment if cyclohexane and cyclohexene are tested. However, the Organic comboplate is manufactured from a material which is resistant to organic solvents and is recommended for this experiment, because alkanes and alkenes other than cyclohexane and cyclohexene may dissolve the plastic of the conventional comboplate. Gloves should be worn throughout the experiment to protect the skin from the corrosive nature of the chemicals used. The teacher must carry out a risk assessment to decide whether protective glasses should be worn by the students. The teacher must also ensure that a waste container is provided into which all organic waste can be disposed. 3. Hints Students should be advised to label their propettes containing cyclohexane and cyclohexene as these are both colourless and can easily be confused. Two or three students can share propettes of the alkane, alkene, bromine solution and potassium permanganate solution because only a few drops of each is required. Cyclohexene (and solutions of bromine in carbon tetrachloride), tend to flow easily from the tip of a propette. Propettes containing these liquids should never be pointed upwards. Bromine solutions are decomposed by heat and light. Light also initiates the substitution reaction between bromine and an alkane, where a hydrogen atom on the alkane molecule is substituted with a bromine atom. Hydrogen bromide (a gas) is produced. The bromoalkane product is colourless, leading the student to the conclusion that the alkane has reacted with the bromine and is therefore an unsaturated compound. To avoid such situations, the bromine solutions should be protected from light at all times. Students should be advised to share propettes of the bromine solution so that the bottle containing the solution can be stored away in a cool, dark place for the remainder of the experiment. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 41 Note that the bromine solution in the propette may also fade during the activity and it may be necessary to use a room with subdued lighting while carrying out the experiment. This can be achieved by switching off overhead lights or blocking direct sunlight from external sources, such as closing curtains or dropping blinds at the windows. The student worksheet suggests that the cyclohexane-bromine and cyclohexene-bromine mixtures are left in the comboplate until after Baeyer’s Test. The objective of leaving the mixtures for an extended period of time is to show the effect of light on the cyclohexane-bromine mixture. The latter is colourless at the end of the lesson because of the substitution of cyclohexane by bromine. There are some questions after Part 2 which intend to help make the substitution reaction known to the student, and to show that this photo-reaction is not an indication of the presence of an unsaturated hydrocarbon compound. When an aqueous solution of bromine is added to both the cyclohexane and cyclohexene, two layers are observed in each well because non-polar organic compounds are immiscible with water. Stirring the contents vigorously disperses the two layers into a suspension. Bromine does not react with cyclohexane and as a result, the contents of the well containing cyclohexane appear brown in colour. After a while, two layers separate out again and the lower layer remains brown in colour. If the comboplate is left to stand for longer than about 3 minutes, the brown colour of the bromine begins to fade as a result of the substitution reaction explained previously. It is therefore important for students to make observations and record results soon after stirring of the well contents. During the stirring of the bromine and cyclohexene layers, the bromine reacts with the alkene and the brown colour fades as the colourless addition product (1,2-dibromocyclohexane) is formed. With standing, two layers may still be evident but the brown colour of the lower layer continues to fade as the bromine is used up. After 1 to 3 minutes, the bromine is either pale brown or colourless. The latter is the usual observation. The time taken for the bromine to become colourless is also dependent on the concentration of aqueous bromine test solution used. If the bromine solution is pale brown (low concentration of Br2 molecules) at the start of the experiment, less time will be needed for the brown colour to disappear than if the bromine solution is dark brown (high concentration of Br2 molecules). If a solution of bromine in carbon tetrachloride is used instead of an aqueous bromine solution, the student should notice that when the Br2/CCl4(l) is stirred with the cyclohexane, the entire solution appears brown in colour. If water is present in the alkane to be tested, two layers will be evident. The lower layer is brown in colour. As with the aqueous bromine solution, the brown colour also fades with time because a substitution reaction slowly occurs between the bromine and alkane. Adding a solution of bromine in carbon tetrachloride to cyclohexene results in immediate decolourisation of the bromine. (As a drop of bromine solution enters the cyclohexene, the brown colour disappears.) There is therefore no agitation required because they are miscible. Note that the model answers contain results obtained with Br2(aq) only. In Baeyer’s Test for unsaturation, the test is only positive if an excess of the potassium permanganate solution turns brown. For this reason the volume of potassium permanganate used is double that of the alkane or alkene to be tested. When cold 0.1% potassium permanganate solution is added to cyclohexane, two layers are seen with the purple colour in the lower aqueous layer. Stirring the contents of the well disperses the two layers, but these separate again. The lower layer remains purple as there is no reaction between the permanganate and alkane. The permanganate changes colour from purple to brown within a few seconds of adding the solution to cyclohexene (seen as a dark brown, lower layer in the well). Stirring is not essential but often helps to show the colour change better. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 42 Other alkanes and alkenes, as well as alkynes, can be tested with the bromine and potassium permanganate solutions. The Organic comboplate should be used if the effect of such compounds on the plastic of an ordinary comboplate is not known. It is important to apply both tests to each compound because some alkenes react slowly under the conditions of the bromine test. The mixtures in both parts of the experiment are easily discarded into a waste container and the comboplate can be rinsed clean with water (assuming the use of aqueous bromine and permanganate solutions). 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Cyclohexene and the solution of bromine in carbon tetrachloride tend to flow easily from the propette tip. Avoid dropping any of the organic reagents onto the skin. If any of these chemicals is spilled onto the skin, wash the affected area with copious amounts of water. Do not allow any flames to be brought near the organic reagents used in this experiment. They are all flammable (with the exception of carbon tetrachloride if it is used). Do not inhale the vapours of the cyclohexane, cyclohexene and bromine solutions as they are poisonous and may cause respiratory damage. Keep the bottles of all organic liquids closed so as to minimise vaporisation. Note that carbon tetrachloride is extremely dangerous. The bromine solutions are corrosive. Avoid dropping the bromine solution onto skin or fabrics. If contact is made with the skin, wash the affected area with copious amounts of water. Keep the bottle containing the bromine solution in a cool, dark place as it decomposes in the presence of light and heat. (Bromine has a high vapour pressure: the vapour above the solution will expand with an increase in temperature.) Potassium permanganate solutions are poisonous. Rinse your hands thoroughly if contact is made with the skin. Dispose of all organic waste in the waste container provided, and not down the drain! MODEL ANSWERS Part 1: The Bromine Test for Unsaturated Hydrocarbon Compounds Q1. What happens when the bromine solution is first added to each compound? A1. Two layers can be seen in each well when the Br2(aq) is added to the cyclohexane and cyclohexene. The lower, aqueous layer in each well is brown in colour. Q2. What do you notice when the bromine solution is stirred with each compound? A2. The bromine remains brown in the well containing the cyclohexane, even with vigorous stirring. The aqueous solution of bromine begins to lose colour during stirring with cyclohexene and appears pale brown in colour. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 43 Q3. Prepare a table like Table 1 below. Record your observations for wells A1 and A2. A3. TABLE 1: TESTING OF SATURATED AND UNSATURATED COMPOUNDS WITH BROMINE SOLUTION COMPOUND Cyclohexane Cyclohexene APPEARANCE OF WELL CONTENTS AFTER 1 MINUTE REACTION WITH BROMINE (Br2 (aq))? There are tw o layers. The bromine in the low er layer has retained its brow n colour. No There are tw o layers. The low er layer is pale brow n or colourless. Yes Q4. Write down the structure of cyclohexane. Is the compound saturated or unsaturated? Explain your answer. cyclohexane, C6H12 A4. The cyclohexane is saturated. There are only carbon-carbon and carbon-hydrogen single bonds in the molecule. One molecule of the compound has the maximum number of hydrogen atoms per carbon atoms. Q5. Write down the structure of cyclohexene. Explain whether the compound is saturated or unsaturated. cyclohexene, C6H10 A5. The cyclohexene is unsaturated. A molecule of the compound contains one carboncarbon double bond. The double bond replaces two possible carbon-hydrogen bonds i.e cyclohexene molecules have two hydrogen atoms less than cyclohexane molecules. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 44 Q6. Based on your answers to questions 4 and 5 above and on the results of the bromine test in your table, describe how mixing a bromine solution with a hydrocarbon compound of unknown structure can help one to tell whether it is saturated or unsaturated. A6. Saturated hydrocarbon compounds like cyclohexane do not react with bromine solution so that the bromine retains its brown colour. Unsaturated hydrocarbon compounds like cyclohexene react with bromine solution so that the bromine is decolourised. If a hydrocarbon compound of unknown structure is mixed with a bromine solution and the brown colour disappears, the compound is unsaturated. If the brown colour of bromine remains, the compound is saturated. Q7. Write down a balanced equation for the reaction of bromine with cyclohexene. Is the product of the reaction saturated or unsaturated? Explain your answer by describing what type of reaction is involved. A7. H Br H H H H H H H H H + Br2 H Br H H H H H H H H cyclohexene, C6H10 H 1,2-dibromocyclohexane, C6H10Br2 The product, 1,2-dibromocyclohexane, is saturated. An addition reaction has occurred i.e the bromine molecule has added across the double bond of the cyclohexene molecule. Q8. Explain why the brown colour of bromine disappears when it is mixed with cyclohexene. (Hint: examine the equation written for question 7 above.) A8. The bromine molecules are used up in the addition reaction. The product of the addition reaction (1,2-dibromocyclohexane) is colourless. The decrease in concentration of brown bromine molecules and the increase in the concentration of the colourless product molecules causes the mixture to fade, and eventually become colourless as all the bromine molecules are consumed. Q9. The bromine addition test does not distinguish between a double or triple bond. The bromine always adds across the multiple bond to form a trans addition product/s. Write down the equation/s for the reaction/s involved when bromine is mixed with ethyne (acetylene). A9. Ethyne 1,2-dibromoethene tetrabromoethane The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 45 Part 2: Baeyer's Test for Unsaturation: Reaction of Cold, Dilute Potassium Permanganate solution with Unsaturated Hydrocarbon Compounds Q1. What do you notice in each well immediately after adding the potassium permanganate solution? A1. There are two layers in each well. The lower aqueous layer is purple in the well containing the cyclohexane, but the lower layer in the well with the cyclohexene is already changing colour to brown. Q2. Describe the appearance of the cyclohexane-permanganate mixture in well A4 after stirring. A2. There are still two layers. No colour change has occurred in the lower layer i.e it has remained purple. Q3. What has happened to the cyclohexene-permanganate mixture after stirring? A3. The lower layer has changed in colour from purple to dark brown. (The permanganate actually decolourises immediately but the colour change is often seen better with stirring.) Q4. How can a dilute potassium permanganate solution help the chemist to distinguish between saturated and unsaturated hydrocarbon compounds? A4. Unsaturated hydrocarbon compounds react with potassium permanganate, causing the solution to change colour from purple to brown. Saturated hydrocarbon compounds do not react with the permanganate and it is therefore not decolourised. Q5. If you have been able to leave the bromine-cyclohexane and bromine-cyclohexene mixtures from Part 1 in the comboplate® until the end of the lesson, answer the questions which follow: i. What do you notice about the appearance of the bromine-cyclohexane mixture? ii. If cyclohexane is a saturated hydrocarbon compound, why has the brown colour faded? Use the following reaction equation to explain your answer. iii. A5. Name the kind of reaction shown in the given equation. i. It is colourless (or very pale brown in colour). ii. One hydrogen atom on the cyclohexane molecule has been substituted with one bromine atom from the bromine molecule. The bromocyclohexane product is colourless and hydrogen bromide is a gas. The increase in concentration of the colourless product and the simultaneous decrease in the concentration of brown bromine molecules causes the mixture to decolourise. The reaction is initiated by light. iii. A substitution reaction. (One atom is substituted with another kind of atom.) The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 46 Q6. The figure below shows a scheme for testing hydrocarbon compounds to determine whether they are saturated or unsaturated. Draw the scheme and complete it by filling in the missing words related to the experiments completed in the worksheets of Parts 1 and 2. A6. Note: that even if the results of both the bromine and Baeyer's tests are positive, there remains an element of doubt. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 47 INVESTIGATING INTERMOLECULAR FORCES IN ORGANIC COMPOUNDS BY MEASURING THE BOILING POINT TEACHER'S GUIDE AND MODEL ANSWERS Introduction The boiling of organic compounds involves the weakening or the breaking of intermolecular forces (or Van der Waal's forces) between molecules. The temperature of boiling of a substance is determined by the kinds and magnitude of intermolecular forces between molecules. There are two types of intermolecular forces: London forces - these are weak instantaneous forces due to distortion of electron clouds. Dipole-dipole forces - these are a result of permanent polarity in molecules leading to stronger attractive forces. Hydrogen bonding is a special type of dipole-dipole force. It occurs when a hydrogen atom is bonded to a highly electronegative F, O or N atom. Because it is stronger than most other intermolecular forces, its presence can significantly increase the boiling point of a substance. 1. Chemicals All the chemicals required are found in the requirements section preceding the experiment. In this investigation, ethanol and 1-butanol will be used as examples. 2. Apparatus The vials, propettes, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry Kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the conventional comboplate can be affected by the organic reagents. 3. Hints You may want to underpin this experiment with a lesson on intermolecular forces (hydrogen bonding, dipole-dipole forces, and induced-dipole forces). Two or three students can share one propette to dispense the 1-butanol because only five drops are used per student. When heating the fusion tube to evaporate 1-butanol, be very cautious that you don't melt the retort stand. If you have a suitable metal replacement, use that instead. You can even use a wooden peg to hold it over the flame. Don't leave the glass vial over the hot flame continuously for more than a minute or two without moving it around to change the position of the flame. If the flame is concentrated to one spot on the vial for too long this can cause the glass to crack and the vial to break. Always extinguish the flame of the microburner after a boiling point determination and let the glass cool. Do not leave the burner under the vial. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 48 4. Cautions (Information from Biggs, P. The Physical and Theoretical Chemistry Laboratory: Chemical and Other Safety Information. Oxford University http://physchem.ox.ac.uk/MSDS/#MSDS). Methylated spirits is a mixture of ethanol (95%) and methanol (5%). Methanol is toxic by inhalation, ingestion or skin absorption. Use gloves in a well-ventilated room. Ethanol can cause nausea, vomiting and inebriation upon ingestion, as well as skin and eye irritation. Work in a wellventilated room with gloves. 1-butanol: Is a harmful substance when inhaled, ingested or when absorped into skin. It is an irritant and a narcotic which is a CNS depressant. Work in a well-ventilated place, with safety glasses and gloves. Keep it away from any sources of ignition. MODEL ANSWERS TO QUESTIONS Q1. Report the observed boiling points for ethanol and 1-butanol. Q2: Why is it important that the thermometer and fusion tube do not touch the bottom of the glass vial. A2: The glass vial is in direct contact with the flame, and thus its temperature is likely to be higher than the oil that it contains. Convection in the oil ensures a fairly uniform temperature for objects immersed in it. Q3: Why do bubbles emerge from the capillary tube ? Explain why the boiling point is recorded when they stop. A3: The vapour pressure of a liquid increases with increasing temperature. When the vapour pressure is equal to the atmospheric pressure, boiling occurs (and bubbles emerge). Boiling stops (escape of bubbles stops) when the temperature of the oil falls below the boiling point. Some bubbles may escape from the tube at temperatures well below boiling point. Initially this is due to expansion of air in the capillary tube as the temperature rises. Q4: Why is it necessary to record the atmospheric pressure ? A4: Since boiling occurs when the vapour pressure is equal to the atmospheric pressure, the boiling point depends on the atmospheric pressure. Hence, atmospheric pressure should always be recorded when reporting a boiling point. Tables of reference books usually give boiling points at 1 atm/760 mm pressure. Q5: Draw out the line drawings for 1-butanol and ethanol. A5: Q6: What kinds of intermolecular forces occur between the 1-butanol molecules and between the ethanol molecules. A6: Hydrogen bonds, dipole induced dipole bonds and Van der Waal's dispersion forces. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 49 Q7. Explain the difference in boiling points between 1-butanol and ethanol in terms of the intermolecular forces. A7: The magnitude of the dispersion forces between molecules depends on the nature and number of the atoms in the molecules. Comparing the molecules of ethanol and 1butanol, the atoms are the same kind in both (C, H, O). However the number of atoms is greater in 1-butanol molecules and hence larger intermolecular forces occur. EXTENSION QUESTION Q8. Predict the boiling point of 1-propanol, giving an explanation of your prediction. A8. The boiling point of 1-propanol should be approximately the average of those of ethanol and 1-butanol. As explained previously, the atoms in 1-propanol molecules are the same as those in ethanol and 1-butanol molecules. The number of these atoms (12), is intermediate between the number in ethanol (9) and 1-butanol (15) molecules. Hence approximately the intermolecular forces between 1-propanol molecules are intermediate in strength and the bp is midway between that of ethanol and 1-butanol, ie. 1/2(118+78) = 98oC. The literature value is 97oC. Note that ethanol, 1-propanol and 1butanol are members of a homologous series (the normal alcohols) and that it is typical of such a series that there is a gradual, regular trend in all physical properties along such a series. Q9. Predict the boiling point of 2-methyl-2-propanol, giving an explanation of your prediction. A9. 2-methyl-2-propanol is a structural isomer of 1-butanol. Its molecules therefore have the same atomic composition as those of 1-butanol but they are arranged in different sequences within the molecule. This is shown in the line drawings: Because they are structural isomers, the intermolecular forces are of the same kind in the two. Also because the atomic composition is the same the total number of electrons is the same. However, the structural differences imply that the molecules cannot interact with their neighbours in the same way. The molecules of 2-methyl-2propanol are more compact and ball-shaped: hence they have a lesser surface area of contact with neighbouring molecules. This means a lesser overall intermolecular force and hence a lower boiling point for 2-methyl-2-propanol. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 50 MELTING POINT DETERMINATIONS TEACHER'S GUIDE AND MODEL ANSWERS Introduction The melting point of any particular organic substance is a very useful property since it can help the organic chemist to determine the purity and identity of the compound in question. Pure organic substances normally have sharp melting points, although some solid compounds do not. It is a general rule that the addition of an impurity to a pure compound lowers its melting point. 1. Chemicals All the chemicals required are found in the requirements table preceding the experiment. 2. Apparatus The vials, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry Kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the conventional comboplate can be affected by the organic reagents. The thermometer is used in the combostill kit. 3. Hints You may want to underpin this experiment with a lesson on intermolecular forces (hydrogen bonding, dipole-dipole forces, induced dipole forces). Don't leave the glass vial over the hot flame continuously for long periods of time (30-60 mins) as this will cause the glass to get too hot and crack. 4. Cautions Benzoic acid is harmful if swallowed, can act as an eye or respiratory irritant and may cause allergic respiratory or skin reaction. Wear safety glasses and gloves when using it. Methylated spirits is a mixture of ethyl alcohol (95%) and methyl alcohol (5%). Methanol is toxic by inhalation, ingestion or skin absorption. Use gloves in a well ventilated room. Ethanol can cause nausea, vomiting and inebriation upon ingestion, as well as skin and eye irritation. Work in a well ventilated room with gloves. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 51 MODEL ANSWERS Q1: Look up the melting point of benzoic acid in the literature. Compare it to your melting point and explain any differences. A1: 122 oC. Differences may be due to impurities. It also may be due to inaccurate thermometers (since we didn't calibrate them). Q2: Explain why the amount of sample in the capillary tube should be kept as small as possible for a melting point determination. A2: The more sample in the capillary tube, the more heat is required to melt all the sample. If too much sample is placed in the capillary tube, it won't all melt uniformly, it will melt over a period of time. The less sample in the tube, the more likely the melting point will be reached instantaneously and the more likely the reading will be made accurately. Q3: A3: Draw out the line drawing for benzoic acid. Q4: What kinds of intermolecular bonds occur between the benzoic acid molecules? A4: There are hydrogen bonds between the -OH groups and dipole-dipole forces between C=O groups. There are also instantaneous dipole-dipole forces between the hydrocarbon parts of the molecules. Q5: Do you think that the melting point of benzene will be higher or lower than that of benzoic acid ? Explain your reasonings. A5: Higher, because there are no hydrogen bonds, only instantaneous dipole-dipole forces. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 52 CHAPTER 2 General Procedures 53 General Procedures 1. Assembling the Wire Microvial-holder 55 2. Vacuum Filtration and Washing of the Collected solid 56 Making Capillary tubes for Melting and Boiling Point Determinations 58 4. Boiling Point Determinations 58 5. Melting Point Determinations 60 6. Making TLC Spotting Capillary Tubes 62 7. Thin Layer Chromatogram (TLC) 63 3. 54 GENERAL PROCEDURES The general procedures are experimental procedures or techniques that can be used in many, many experiments (especially organic chemistry ones) using the RADMASTE microchemistry kits. 1. Assembling the Wire Microvial-holder Apparatus: Organic comboplate, lid 1, glass vial, elastic band, microburner, iron wire (15 cm x 1.6 cm diameter). 1. Curl the 15 mm long piece of iron wire around the center of the glass vial, so that it forms a loop that fits exactly around the outside of the vial. 2. Place lid 1 into well E1. 3. Place the other end of the wire in the small opening in lid 1 so that it is touching the bottom of the comboplate as shown in the figure, and bend it at the end of the loop so that it can hold the glass vial vertically. 4. Wrap a piece of elastic around the centre of the glass vial and place it in the loop of the wire vial-holder. 5. Place the microburner under the vial. 6. Make sure the wire is bent in such a way so that the vial is about 2-3 mm above the flame of the microburner. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 55 2. Vacuum Filtration and Washing of the Collected Solid microstand in well D5 of comboplate Apparatus: Organic comboplate, lid 1, glass vial with solid-liquid mixture to be filtered, silicone tubing, plastic microburner vial, cotton wool (or string), microfunnel, paper clip, syringe, glass rod, plastic microretort stand. 1. Unfold a paper clip. 2. Place a small piece of cotton wool into the opening of the microfunnel. 3. Force the cotton wool into the stem of the microfunnel using an unfolded paper clip. (In some experiments, string is used instead of cotton wool because cotton wool is attacked by the chemicals). Ensure that there is enough cotton wool in the funnel and that it is tightly wedged into the stem of the funnel so that it fills the opening ensuring that all the liquid has to pass through the cotton wool upon filtration. If you are using string, tie a double knot in it with both knots on top of one another. Then use a pair of scissors to cut the two loose ends of the string off. 4. Place the plastic microburner vial (without the lid) into well F1 in the comboplate and place lid 1 into it. 5. Place the syringe into the larger opening in lid 1. 6. Place a piece of silicone tubing over the smaller opening of lid 1. 7. Place the stem of the microfunnel into the other end of the silicone tubing. 8. Place the plastic microretort stand into well D5 of the comboplate and clamp the silicone tube into the arm just below the stem of the microfunnel so that it is not touching the syringe. This is to hold the funnel upright and to make sure that it is steady during filtration. 9. Make sure that the microfunnel is held as nearly perpendicular as possible so as to minimise spilling of liquid during the filtration. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 56 10. Make sure all the connections are sealed tightly otherwise no vacuum will be produced. 11. Slowly pour the solid/liquid mixture in the glass vial into the microfunnel until it is ¾ full. 12. Slowly pull the plunger out of the syringe (this creates a vacuum in the plastic microvial), drawing the liquid out of the microfunnel into the plastic vial leaving the solid residue in the microfunnel. 13. Repeat this process until all the solid has been transferred from the vial to the funnel. 14. Use a glass rod to scrape any solid remaining in the vial into the funnel. 15. You can also use a little water (or suitable solvent) (about 0.1 ml) to get any solid that sticks to the sides of the vial into the microfunnel. 16. Having filtered all of the mixture, add a further 0.5 ml water (or other suitable solvent) to the original container to wash out into the funnel any crystals that are still adhering to its sides. Filter these washings through the funnel using the syringe. The aim is to get as much of the solid into the funnel as possible. 17. Use a microspatula (or a glass rod) to scrape any remaining crystals out of the original container into the microfunnel. 18. Continue this process until all the solid has been collected in the microfunnel. 19. Some of the original liquid may adhere to the surface of the crystals in the funnel. This can be washed away using a little water (or other suitable solvent), and then continuing the vacuum filtration. 20. Draw up a small amount of water (or other suitable solvent) - about 1 ml - into the syringe or a propette. 21. Slowly add it to the funnel containing the solid. 22. Using the suction action of the syringe, draw out this liquid from the funnel. 23. The solid has now been collected and washed. 1 If the plunger is completely pulled out before all of the liquid has been drawn from the microfunnel, remove the syringe from lid 1, and push the plunger completely in. Replace the syringe in lid 1 and draw the plunger out slowly again to continue filtration. 2 The microburner vial may become full, at some point. This means that no more liquid will be suctioned from the funnel. If this is the case, slowly remove the lid from the vial and empty its contents into the organic waste container - make sure you don't lose any crystals in the process - and then replace the lid. 3 Note the difference between ordinary 'gravity' filtration and vacuum filtration. Gravity filtration relies on the force of gravity to draw the liquid through the filter paper into the collection vial. This however, is not always effective, because solid can clog up the filter causing the liquid phase to be unable to pass through the filter paper (ie. the force of gravity is not strong enough to draw the liquid through the filter paper because of the collected solid that clogs it) and the filtration process can take a very, very long time. Vacuum filtration speeds up the process generating an additional force to gravity that draws the liquid through the filter. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 57 3. Making Capillary Tubes for Melting and Boiling Point Determinations 1. Light the microburner flame and hold the one end of a capillary tube horizontally, directly into the flame (see figure 1). The glass should begin to melt and the end of the capillary tube should close. 2. As the glass is melting, twirl the capillary tube between your fingers to make sure it remains straight as it melts. 3. Once the end of the capillary tube is totally sealed, pull it out of the flame and let it cool to room temperature. 4. Boiling Point Determinations REQUIREMENTS Apparatus: 1 x Organic comboplate®; lid 1, 1 x glass vial, wire vial-holder, 1 x microburner, matches,1 x paper clip, 2 x elastic bands, 2 x capillary tubes, 1 x 150oC thermometer, 1 x microretort stand, 1 x fusion tube, 1 x propette, 3 capillary tubes, tissue paper. Chemicals: Boiling chips, paraffin oil, methylated spirits, sample. PROCEDURE 1. Take a capillary tube sealed at one end. (see General Procedures, section 3). 2. Place lid 1 in well E1 of the comboplate. 3. Assemble the wire vial-holder (see General Procedures, section 1). 4. Place the wire vial holder into the small opening in lid 1 so that it is touching the bottom of the well (see figure 2). 5. Wrap the elastic band around the center of the glass vial, so that it fits tightly. 6. Fill the glass vial with paraffin oil to a depth of approximately 1 cm and place 2-3 boiling chips into the oil. 7. Rest the glass vial in the wire vial-holder. 8. Clip the thermometer into the microretort stand and attach the fusion tube with an elastic rubber band. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 58 9. Place the microretort stand in well D12 and submerge the thermometer and fusion tube halfway into the oil (see figure 3). Do not let the thermometer or the capillary tube touch the bottom of the vial during heating. Also keep the elastic band out of the oil. 10. Using a propette place the liquid sample into the fusion tube until it is ¾ full. 11. Place the open end of the capillary tube into the fusion tube (see figure 3). 12. Light the microburner and place it directly under the vial (see figure 2). Watch the rise in temperature. 13. Bubbles will emerge from the open end of the capillary tube, slowly at first, but eventually almost continuously. When the stream of bubbles is continuous, stop heating by removing the flame of the microburner from beneath the vial. Extinguish the microburner flame. 14. Observe and record the temperature when the evolution of bubbles just ceases as the liquid cools. This is the boiling point of the liquid. Also note the atmospheric pressure. Do this experiment two times. This is because the boiling temperature is unexpected the first time you conduct the experiment and your first measurement may not be read accurately. Thus, conduct this experiment the first time to obtain a rough estimate, then conduct it again to get a more accurate reading. 15. Take the fusion tube out of the oil and allow it to cool to room temperature. You may have to top up the fusion tube with new sample so that it is ¾ full because some of it may have evaporated in the previous boiling point determination. 16. Keep the rate of temperature rise low for the 2nd determination. This is done by removing the microburner from under the oil every few seconds and replacing it. The closer you get to the boiling point, the slower the rise in temperature should be. When you have completed your 2 observations, discard the capillary tubes. Wash the fusion tube with water, clean out the fusion tube with a piece of tissue paper and leave it in the sun to dry. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 59 5. Melting Point Determinations REQUIREMENTS Apparatus: Organic comboplate, lid 1, lid 2, glass vial, wire vial-holder, 1 x microburner, 2 x elastic bands, 2 x capillary tubes, 3 x microspatulas, 1 x microretort stand, 1 x 150oC thermometer. Chemicals: Boiling chips, paraffin oil, benzoic acid (C7H6O2(s)), methylated spirits, unknown solid, matches. PROCEDURE 1. Prepare 2 capillary tubes for a melting point determination (see General Procedures, section 3) 2. Place 4 microspatulas of finely powdered solid into well A1 of the comboplate. 3. Push the open end of the capillary tube through this portion of the finely powdered solid, making sure that some of the solid is pushed into it. 4. Tap the closed end of the tube on the desk so that the solid collects at the sealed end. 5. A height of solid about 3 mm - 5 mm is suitable. 6. Place lid 1 in well E1 of the comboplate. 7. Wrap the elastic band around the centre of the glass vial, so that it fits tightly. 8. Place the wire vial-holder (see General Procedures, section 1) into the small opening in lid 1 so that it is touching the bottom of the comboplate. 9. Rest the glass vial inside the loop. 10. Fill the glass vial with oil to a depth of approximately 1 cm and place 4-5 boiling chips into the oil to prevent the oil from spluttering out of the vial. 11. Attach the capillary tube to the thermometer using a rubber band (See figure 1). Clip the thermometer into the microretort stand and place it in well D2 of the comboplate. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 60 12. Submerge the thermometer and capillary tube halfway into the oil. Do not let the thermometer or the capillary tube touch the bottom of the vial. Do not let the elastic band touch the oil. 13. Light the microburner and place it directly under the vial. 14. Continue to hold the vial over the flame until the solid melts. 15. Note the temperature at which the solid suddenly melts in the tube. 16. Discard the capillary tube. Repeat this experiment to obtain a second, more accurate result. This is because the melting temperature is reached fairly quickly and your first measurement may not be read accurately. Thus, conduct this experiment the first time to obtain a rough estimate of the melting point. Then conduct it again to get a more accurate result. 17. Keep the rate of temperature rise low for the 2nd determination. This is done by removing the microburner from under the oil every few seconds and replacing it. The closer you get to the melting point, the slower the rise in temperature should be. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 61 6. Making TLC spotting capillary tubes 1. Light the microburner. 2. Hold the middle of a capillary tube about 3 mm over the flame and rotate it with your fingers so that the heat is evenly distributed. 3. The center part of the capillary tube will become supple from the heat. 4. At this point quickly pull it out of the flame and pull the two ends away from each other making the middle part long and thin. Break here Don't hold the tube over the flame too long or it will split in half because of the heat before you have drawn it out. 5. Break the extended tube in half. 6. Each half is a TLC spotting capillary tube. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 62 7. A Thin Layer Chromatogram (TLC) Do not touch the surface of the TLC plate with your fingers. Hold it on it's sides only - like a photographer would hold a photographic plate so as not to get any finger marks on it. 1. Draw a pencil line across the TLC plate about 10 mm from the bottom. Do not use an ink pen, the ink will run and make the spots difficult to see. 2. Draw as many pencil dots at evenly spaced points across the plate as substances there are to be analysed. These dots are where you spot the TLC plate. 3. Label each dot according to the substance you plan to spot there in pencil. 4. Draw out as many TLC spotting capillaries as spots to be made on the plate (see General Procedures, section 6). 5. Place 1 microspatula of each solid to be spotted into one of the wells of the comboplate. 6. Using a propette, place enough solvent into each well so as to dissolve the solid. 7. Dip a spotting capillary into well E1; it should draw up some of the liquid. 8. Gently dab the end of the capillary onto the TLC plate in the place where you wish the spot to be. A sp .B A 9. N B Do this for each of the substances you wish to analyse. Do not make the spot too big by holding the capillary tube against the TLC plate for too long. A diameter of no more than 2 mm is sufficient. 10. Fill the glass vial to a height of about 5 mm with the designated developing solvent (which is normally a mixture of substances). 11. Place the TLC plate inside the vial so that the top is resting against the side of the vial. 12. Place the lid upside down on top of the vial. Asp. BA N B The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 63 13. Allow the developing solvent to run up the TLC plate until it is about ¾ of the way up and then remove the plate from the vial. 14. Immediately trace the developing solvent front with a pencil to indicate where it is. This must be done quickly since the solvent will evaporate and the solvent front won't be visible. 15. Allow the plate to dry for some time. 16. Empty the remaining developing solvent into the waste bottle provided. Not down the sink. 17. Wash the vial with water and dry it with a piece of tissue paper. 18. Put one or two pieces of iodine crystals into the vial and put the lid on properly this time. 19. Pour boiling water into the plastic lunch box and hold the vial over in the steaming water for 1-2 minutes. 20. After a while, the presence of iodine vapour will be observable in the vial. 21. Place your TLC plate into the vial in the same way as before. 22. Brown spots begin to form on the plate corresponding to where the iodine has interacted with the substances spotted. 23. After a minute or two remove the plate from the vial and immediately mark the postion of all spots, by outlining them on the plate with a pencil. This is because the iodine fades after some time. 24. Measure the distance from the line drawn at the bottom of the plate to the midpoint of each spot and record it. 25. Measure the distance of the solvent front from the line drawn at the bottom of the plate to the solvent front directly above each spot. 26. Calculate the Rf value of each spot by dividing distance travelled by the spot by the distance the solvent front travelled. 27. Use a table like the one below to report your results. Substance 28. Distance travelled by spot /mm (1) Distance of solvent front directly above spot /mm (2) Rf value = (1)/(2) Draw a rough sketch (similar to the one below) of your TLC plate in your report indicating all distances travelled. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 64 CHAPTER 3 Preparation and Reaction of Organic Compounds 65 Preparation and Reaction of Organic Compounds 1. Preparation and Testing of Ethyne (Acetylene) 67 2. Making Esters – the Synthesis of Aspirin 72 3. Recrystallisation and Purification of Aspirin 76 4. The Oxyacetylene Flame 78 5. Acid-base Extraction of Benzoic Acid from Naphthalene 81 Using Esters: Saponification 85 6. 66 PREPARATION AND TESTING OF ETHYNE (ACETYLENE) TEACHERS GUIDE AND MODEL ANSWERS Introduction Calcium carbide is produced industrially by heating a mixture of calcium oxide and coke to 20000 C in an electric furnace. It is then treated with water to produce ethyne (acetylene) gas: CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(s) Ethyne belongs to the alkyne group of hydrocarbons i.e the molecule contains a carbon-carbon triple bond and is represented as HC CH. Electrons which form part of this bond react readily with certain reagents. Bromine and chlorine add to ethyne to give addition products: Solutions of bromine in water and in carbon tetrachloride are brown in colour. As the bromoaddition products are formed, the concentration of bromine molecules in these solutions decreases and the solutions eventually become colourless. This is one method of detecting the presence of ethyne gas. When ethyne is reacted with powerful oxidising agents such as potassium permanganate, the triple bond undergoes oxidative cleavage to form methanoic acid (formic acid) and carbon dioxide: The purple KMnO4 (Mn(VII) is reduced to the orange/brown MnO2 (Mn(IV)). This reaction can also be used to show the presence of ethyne gas because a solution of potassium permanganate in propanone will change from purple to brown when ethyne gas is bubbled through it. Ethyne was previously used as the starting material for the preparation of acetaldehyde, acetic acid, vinyl chloride and some other chemicals. Today it is exploited mainly for its flammability and is burned together with propyne and oxygen in oxyacetylene torches for welding and steel cutting. It is also still used in the preparation of acrylic polymers. 1. Chemicals All the chemicals required are listed in the requirements table preceding the experiment. Calcium carbide samples should be as fresh as possible. Older samples may not have the desired reactivity because water in the atmosphere may have already reacted with the powder. The solution of potassium permanganate in propanone must be freshly prepared by adding a very small quantity (use the narrow end of a microspatula) of solid potassium permanganate to approximately 50 ml of propanone (acetone) with stirring. The solution should preferably be stored in an amber bottle and protected from heat and light. Such a solution is dark purple in colour and an appreciable colour change will not be seen during the experiment unless the solution is diluted to a pale violet colour. The extra propanone is therefore required to dilute the potassium permanganate solution. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 67 Alternatively, a dilute solution of bromine in water or carbon tetrachloride can be used to detect the presence of the ethyne triple bond. If bromine solutions are too dark in colour, they will also need to be diluted until they are pale brown. Water is used to dilute the aqueous bromine solutions and extra carbon tetrachloride is required to dilute solutions of bromine in carbon tetrachloride. 2. Apparatus The propettes, syringe, lids, silicone tubing, microspatulas, glass tube and arms of the microstand are all components of the RADMASTE Microchemistry kits. The organic comboplate® is manufactured from a material which is resistant to organic solvents and must be used in this experiment, because the reaction between calcium carbide and water is so vigorous that the plastic of the conventional comboplate® will crack around the large wells being used. Propanone will also destroy the plastic of a conventional comboplate®. The teacher must carry out a risk assessment to decide whether gloves and protective glasses should be worn by the students. The teacher must provide a waste container into which organic waste can be disposed of. 3. Hints The teacher should ideally perform the experiment prior to the practical session to assess the reactivity of the calcium carbide sample. Samples which are old or have been exposed to the atmosphere will not be as reactive as those that are fresh. Often, it is not the entire sample which is unreactive but rather the first one or two centimetres of powder close to the surface of the sample. The teacher should then instruct the students to remove this quantity of powder from the chemical bottle/s and dispose of it safely. The experimental procedure requires 3 to 5 microspatulas of calcium carbide powder for a reaction that produces a sufficient volume of ethyne gas. If the sample is fresh, only 3 microspatulas will be needed. However, if the reaction with water is poor then a quantity in excess of 5 spatulas of powder may be added. Caution must be exercised here because if too great a quantity of a relatively fresh sample is used, the reaction in F1 will be too vigorous and will cause some of the reaction mixture to flow through the silicone tube into F2. Make sure that the comboplate® and the microspatula used to add the calcium carbide powder are both dry, as the powder will react immediately with any water it comes into contact with. The potassium permanganate solution must be diluted with propanone as described in the worksheet to give a pale violet/pink colour. Such a solution will be sufficiently reduced so that a pale brown-coloured mixture remains after ethyne gas has been bubbled through it. If the potassium permanganate solution is not diluted, the volume of ethyne gas produced from the reaction in F1 will be too little to cause any appreciable colour change when bubbled through the solution. Similarly, bromine solutions need to be diluted until they are pale brown in colour otherwise they will not be colourless at the end of the experiment. Two or three students can share one propette of potassium permanganate or bromine solution because only five to ten drops of each liquid is required. Well F2 should not be filled to more than b of its capacity, otherwise solution will spill out of the vent in lid 2 as ethyne gas bubbles rapidly through it. Ensure that the wells are securely sealed. If the lids are not tight, ethyne gas will escape from the wells and the desired reactions may be incomplete. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 68 When adding the water from the syringe, it may sometimes happen that the water does not drop directly onto the powder in the well but collects on the inside of lid 1. For this reason, only about 0,1 ml of water must be added initially. If the water does not immediately drop onto the calcium carbide powder, the top of the syringe must be tapped gently to force the water into the well from the inside of the lid. The remainder of the water must then be added slowly from the syringe to prevent the contents of F1 from being pushed through the silicone tubing into F2. Students may observe the escape of a white, foul smelling vapour from the vent in lid 2 during the experiment. Remind them that ethyne is not foul smelling and that these observations are due to an impurity found in the calcium carbide. During the experiment it is normal for the lids to swell slightly, and it may be difficult to remove them afterwards. The swelling subsides after some time. It is however important to prevent solution being sucked back from well F2 into well F1 as soon as the bubbling in well F2 ceases. If the lid on well F2 fits too tightly to be removed, the silicone tubing connecting lids 1 and 2 can be removed instead to avoid suck-back. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Do not inhale the ethyne vapour deeply! The vapours are easily detectable. Serious respiratory damage and dulled receptiveness may occur if this rule is not adhered to. Never bring a flame near any of the organic reagents used in this experiment, including the calcium carbide powder. They are all flammable. Extinguish the flame of the microburner as soon as you have finished using it. It is obvious from this experiment that calcium carbide reacts violently with water. Make sure that the sample is kept dry at all times and store the powder in a screw top bottle. Bromine has a high vapour pressure i.e. the vapour above the solution will expand with an increase in temperature. Bromine solutions must therefore be kept in a cool, dark place in well stoppered bottles. The halogens are corrosive. Avoid dropping the bromine solution onto fabrics and skin. Wash hands thoroughly if any bromine solution makes contact with the skin. Carbon tetrachloride and the bromine solutions form toxic vapour. Avoid inhaling these vapours and make sure the experiment is performed in a well ventilated room. Dispose of all organic waste in the waste container provided, and not down the drain! The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 69 MODEL ANSWERS TO QUESTIONS Q1. What is the colour of the potassium permanganate/propanone solution? A1. Pale violet/pink. Q2. What happens in well F1 when a drop of water falls onto the calcium carbide powder? A2. A vigorous, rapid reaction occurs. A fizzing sound is heard and there is bubbling where the water makes contact with the powder. Q3. What is happening in well F2? A3. Bubbles appear rapidly in the solution in F2. Q4. Has the appearance of the potassium permanganate/propanone solution changed? Describe. A4. Yes. The potassium permanganate/propanone solution has changed colour from pale violet to a pale orange-brown. Q5. Observe the colour of the indicator when added to the residue in well F1. A5. The indicator colour is purple. Q6. What has happened to the solution in well F2 after about 5 minutes? A6. The solution has become colourless and a brown solid has settled at the bottom of the well. Q7. Write down a balanced chemical equation to explain the reaction that occurred in well F1 between the calcium carbide and water. A7. CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(s) Q8. Use the answer to question 1 to explain the change in colour of the Universal indicator solution in well F1. A8. The calcium hydroxide formed in the reaction is basic and caused the indicator colour to change to purple. Q9. While the gas was bubbling into the solution in F2, the following change took place: KMnO4(aq) → MnO2(s) Purple Orange/brown i. ii. iii. A9. Explain what type of reaction this is. Use the partial equation above to describe why the solution in F2 appeared to change when left for 5 minutes after the reaction was complete. If a bromine solution was used, write down a balanced chemical equation to show the reaction occurring in well F2. Why was a colour change observed? i. ii. iii. This is a redox reaction. The oxidation number of Mn has changed from +7 in KMnO4 to +4 in MnO2. Mn has gained electrons and has therefore been reduced. Manganese dioxide is a solid. When the solution was allowed to stand, the solid brown MnO2 particles sank to the bottom of the well. The bromine and ethyne are involved in an addition reaction. The bromine adds across the triple bond of ethyne. A new product (1,2-dibromoethene) is formed. Further addition of bromine results in the formation of saturated 1,1,2,2 tetrabromoethane. The solution in F2 becomes colourless because there are no bromine molecules left in solution. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 70 Q10. Acetylene gas is highly flammable and gives a very high temperature flame when burned with oxygen. Carbon dioxide and water are produced. i. Write down a chemical equation to show the reaction of oxygen with acetylene. ii. Name the process mentioned above. iii. Identify one industrial use of acetylene gas based on the statement made above. A10. i. ii. iii. 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l ) Combustion. It is one of the components of the oxyacetylene torch used for welding and steel cutting. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 71 THE SYNTHESIS OF ASPIRIN TEACHER'S GUIDE AND MODEL ANSWERS Introduction The addition of excess water in step 6 is to ensure that any excess acetic anhydride is used up. The anhydride reacts with water to form acetic acid. This leaves a dilute solution of acetic acid. Since aspirin is insoluble in cold water, it crystallizes out of solution. 1. Chemicals All the chemicals required are listed in the worksheet. Ice is required as well as boiling water - at least enough to fill every student's lunch box or to fill a communal container eg. a bucket of some sort (see step 3). Instead of ice, students can use cold water to cool the reaction mixture. This can be tap water (depending on the ambient temperature) or cold water from a fridge. 2. Apparatus The vials, propettes, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the comboplate used with aqueous solutions can be affected by the organic reagents. A kettle (or a stove with a pot) may be required to boil the water (see step 2). 3. Hints This experiment should take about 2 hrs. Two or three students can share one propette to dispense the concentrated sulphuric acid because only five drops are used per student. Hot water bath: Don't fill the container of hot water too full, otherwise, the reaction vials will topple into the water - make the water depth about 1-2 cm high. The students need only keep their reaction vials in the water bath for five minutes. Vacuum filtration: Try to get as much aspirin product out of the reaction vial into the funnel as possible with as little water as possible. First try to scrape it out and then use water. If not all the aspirin is retrieved out of the vial after trying to get it out with 0.1 ml water, leave it in the vial. It is not important to get every last grain of aspirin out of the reaction vial, since no quantitative analysis is being conducted. Nevertheless, students should still be encouraged to be as accurate as possible in their experimental method. If a balance is available let the students weigh out their salicylic acid starting material and their aspirin product. Using too much water to wash out the crystals from the reaction vial and to wash the product means that the microburner vial will fill up with water and it will have to be emptied and replaced without spilling any aspirin product from the funnel. Therefore, guard against using too much water in these steps. It also means that some of your aspirin product will dissolve. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 72 4. Cautions (Taken from Biggs, P. The Physical and Theoretical Chemistry Laboratory: Chemical and Other Safety Information. Oxford University http://physchem.ox.ac.uk/MSDS/#MSDS). Acetic anhydride is corrosive and causes severe burns when in contact with the skin. It is harmful if swallowed or inhaled, and contact with the eyes should be avoided. For this cause, the entire experiment should be conducted in a well ventilated room or preferably outdoors. Caution the students to keep their noses far from the reaction vials since inhalation is extremely unpleasant. Small doses, such as used in this experiment are not harmful, nevertheless, caution should be maintained. To completely avoid contact with the skin and eyes, it is advisable to use gloves and safety glasses throughout the duration of the experiment. Salicylic acid is harmful by inhalation, ingestion and skin absorption. It is advisable to wear gloves and safety glasses at all times and avoid breathing its dust. Concentrated sulphuric acid is extremely corrosive, causes serious burns and is highly toxic. It is harmful by ingestion, inhalation, and through skin contact. Avoid breathing the vapor. Upon addition of the sulphuric acid to the acetic anhydride/salicylic acid solution, strong, stinging odours may be evolved. Ensure that the reaction vial is held far from the face at all times during addition of sulphuric acid and afterwards. MODEL ANSWERS Q1. Write out the line drawings for salicylic acid, acetic anhydride and aspirin. A1. Q2. What is the purpose of washing the crystals ? A2. Washing the crystals removes water soluble impurities that might adhere to the surface of the crystals. Q3. Write out a balanced chemical equation for the reaction. A3. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 73 Q4. Explain the use of H2SO4 in the experiment A4. H2SO4 is used as a catalyst. It protonates a carbonyl oxygen atom in the acetic anhydride molecule, activating the carbonyl group toward nucleophilic attack by the OH group of the salicylic acid. A proton transfer then occurs and the loss of a water molecule yields a protonated ester. The loss of the proton then yields a free ester molecule and regenerates the acid catalyst. O O + H CH3 CH3 O H O H + + + CH3 CH3 OH O O O CH3 CH+3 O O H OH O OH O O O CH3 + H O O + OH H3C OH O Q5. Acetic anhydride is added in excess. This excess, however, can react with the carboxylic acid group in aspirin. Which step in the procedure guards against this ? Explain. A5. The addition of excess water in step 6 is to ensure that any excess acetic anhydride is used up. The anhydride reacts with water to form acetic acid which is unreactive to aspirin. This leaves a dilute solution of acetic acid. Since aspirin is insoluble in cold water, it crystallizes out of solution. EXTENSION QUESTION Q6. If 1 microspatula of salicylic acid has a mass of approximately 0.2 g, calculate the mass of aspirin that should be formed. A6. Molar mass salicylic acid = 138 g/mol Molar mass aspirin = 180 g/mol mass of salicylic acid = 0.2 g x 6 microspatula = 1.2 g nsal = msal/Msal = (1.2)/138 = 0.0067 mol The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 74 1 mole salicylic acid forms 1 mole aspirin. masp = nsal x Masp = 0.0067 x 180 = 1.56 g Q7. Assuming you obtained 1 g of aspirin, calculate the percentage yield formed. A7. percentage yield = actual mass of aspirin obtained/theoretical mass of aspirin obtained x 100 = 1.00g / 1.56g x 100 = 0.64 x 100 => 64 % yield Q8. One way to prepare esters is by the reaction of an alcohol with an acid anhydride (as in this experiment). However, they also may be prepared by the reaction between an alcohol and a carboxylic acid or an alcohol and an acid chloride. Predict the product of the following reaction; A8. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 75 RECRYSTALLISATION OF CRUDE ASPIRIN AND TEST OF PURITY TEACHER'S GUIDE AND MODEL ANSWERS 1. Chemicals All the chemicals required are listed in the worksheet. 2. Apparatus The vial, propettes, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the comboplate used with aqueous solutions can be affected by the organic reagents. 3. Hints This experiment takes about 45 minutes to perform. However the aspirin product must be left overnight to dry. If a solid remains after heating the crude aspirin in the water/methylated spirits mixture, filter the hot mixture through the funnel, making sure the reception vial is clean. The solid impurities should remain in the funnel. Remove the impurities, take out the string, and replace it with clean string. Allow the solution to cool to room temperature and continue with step 7. Using too much water to wash out the crystals from the reaction vial and to wash the product means that the microburner vial will fill up with water and it will have to be emptied and replaced without spilling any aspirin product from the funnel. Therefore, guard against using too much water in these steps. 4. Cautions (Taken from Biggs, P. The Physical and Theoretical Chemistry Laboratory: Chemical and Other Safety Information. Oxford University http://physchem.ox.ac.uk/MSDS/#MSDS). Ferric chloride is a relatively stable compound. Contact with the skin, however, can cause burns and it is also harmful if swallowed. Ensure the use of gloves and safety glasses throughout its use. Working with such small amounts in a well ventilated room with gloves shouldn't cause any harmful effects. However, still make sure that all safety precautions are observed. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 76 MODEL ANSWERS TO QUESTIONS Q1. Why are the crystals dried ? A1. Some water molecules may remain in the collected crystals and significantly increase the mass (or change the appearance) of the crystals. The presence of water can also affect the observed melting point of the crystals. Water molecules, however, may be removed over time by evaporation (= drying in air). (Note: Since we are not necessarily measuring the mass of our recrystallised product in this experiment, this step may be omitted from the experimental procedure. However, because it is a useful piece of information for students to assimilate and remember, it has been retained). Q2. Report the melting point of your aspirin product, both the approximate and more accurate observations. Find out the melting point of pure aspirin. How can we use the actual melting point to get an idea of the purity of the aspirin product? A2. Table 1: Melting Point Determination of Aspirin T/ o C m.p determination #1 m.p determination #2 The melting point of aspirin is 138-140oC. Impurities trapped in the aspirin crystal have an effect on the intermolecular bonds. Thus, they can change the melting point of the product. By comparing the actual melting point to the literature melting point, we can determine whether the crystals are pure or not. Not only that, but pure samples of a substance normally have sharp melting points (about 1-2oC), impure samples of the same substance melt over a wider range. Q3. Report the results of your ferric chloride test on your recrystallised aspirin and on pure aspirin. A3. Colour in Ferric Chloride Pure aspirin Orange Crude aspirin Purple Q4. Salicylic acid gives a purple colour with ferric chloride. What does the test show about the presence of salicylic acid in the two aspirin samples? A4. Salicylic acid is not present in the pure aspirin sample but is present in the crude aspirin sample. Q5. Name two possible sources for the presence of the salicylic acid in your recrystallised aspirin. A5. Unreacted salicylic acid can come from the starting materials. It can also be due to some decomposition of the aspirin. Q6. How else could you check the purity of your aspirin product? Explain. A6. You could compare the Rf values obtained from TLC spots of recrystallised aspirin, crude aspirin, salicylic acid and commercial aspirin - four spots on one TLC plate. The aspirin would give a characteristic spot in each case. If there were any salicylic acid in the recrystallised aspirin, there would be a spot with an Rf value similar to that of the salicylic acid spot. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 77 THE OXYACETYLENE FLAME TEACHERS GUIDE AND MODEL ANSWERS Introduction Ethyne was previously used as the starting material for the preparation of acetaldehyde, acetic acid, vinyl chloride and some other chemicals. Today it is exploited mainly for its flammability and is burned together with propyne and oxygen in oxyacetylene torches for welding and steel cutting. It is also still used in the preparation of acrylic polymers. The products formed by burning hydrocarbons depend on the concentration of oxygen and the temperature. If there is excess oxygen and a high temperature, combustion of ethyne is complete and the carbon atom is oxidised from -1 in ethyne to +4 in carbon dioxide. This is the highest oxidation state of carbon: 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l ) However, if the temperature is not high enough or the concentration of oxygen is low, the carbon atoms in ethyne are oxidised to an intermediate oxidation state producing carbon (oxidation state 0) and carbon monoxide (oxidation state +2). This is the incomplete combustion of ethyne. It is a less efficient use of the gas because a smaller amount of oxygen reacts with the carbon and less heat energy is released during the reaction. Carbon monoxide is also a poisonous gas. 1. Chemicals All the chemicals required are listed in the requirements table preceding the experiment. Calcium carbide samples should be as fresh as possible. Older samples may not have the desired reactivity because water in the atmosphere may have already reacted with the powder. 2. Apparatus The propettes, syringe, lids, silicone tubing, microspatulas, glass tube and arms of the microstand are all components of the RADMASTE Microchemistry kits. The organic comboplate® is manufactured from a material which is resistant to organic solvents and must be used in this experiment, because the reaction between calcium carbide and water is so vigorous that the plastic of the conventional comboplate® will crack around the large wells being used. The teacher must carry out a risk assessment to decide whether gloves and protective glasses should be worn by the students. The teacher must provide a waste container into which organic waste can be disposed of. 3. Hints The teacher should ideally perform the experiment prior to the practical session to assess the reactivity of the calcium carbide sample. Samples which are old or have been exposed to the atmosphere will not be as reactive as those that are fresh. Often, it is not the entire sample which is unreactive but rather the first one or two centimetres of powder close to the surface of the sample. The teacher should then instruct the students to remove this quantity of powder from the chemical bottle/s and dispose of it safely. The experimental procedure requires only 1 to 2 microspatulas of calcium carbide powder because the smallest amount of gas will ignite in the flame of the microburner. If the sample is fresh, only 1 microspatula will be needed. However, if the reaction with water is poor then a quantity in excess of 1 spatula of powder may be added. Caution must be exercised here because if too great a quantity of a relatively fresh sample is used, the reaction in F1 will be too vigorous. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 78 Make sure that the comboplate® and the microspatula used to add the calcium carbide powder are both dry, as the powder will react immediately with any water it comes into contact with. Ensure that well F1 is securely sealed. If the lid is not air-tight, ethyne gas will escape from the well and the desired flame may not be obtained. When adding the water from the syringe, it may sometimes happen that the water does not drop directly onto the powder in the well but collects on the inside of lid 1. For this reason, only about one drop of water must be added initially. If the water does not immediately fall onto the calcium carbide powder, the top of the syringe must be tapped gently to force the water into the well from the inside of the lid. The remainder of the water must then be added slowly from the syringe. During the experiment it is normal for the lid to swell slightly, and it may be difficult to remove it afterwards. The swelling subsides after some time. The set-up shown in the worksheets has been chosen so that the student does not have to hold the glass tube in a horizontal position while simultaneously trying to add the water to well F1. The oxyacetylene flame "bursts" from the end of the glass tube where it remains for as long as ethyne is produced in F1. The flame becomes smaller and is eventually extinguished as no more ethyne moves through the tube. The combustion of ethyne in this experiment is incomplete, shown by the small particles of soot (carbon) that remain in the air afterwards. 4. Cautions Ensure that all students are aware of the following safety advice: Never point or hold the tip of a propette containing an organic liquid facing upwards. Unlike aqueous solutions, organic liquids escape readily from the propette stem and can suddenly squirt from the propette if it is held with the tip pointing upwards. Do not inhale the ethyne vapour deeply! The vapours are easily detectable. Serious respiratory damage and dulled receptiveness may occur if this rule is not adhered to. Never bring a flame near any of the organic reagents used in this experiment, including the calcium carbide powder. They are all flammable. Extinguish the flame of the microburner as soon as you have finished using it. It is obvious from this experiment that calcium carbide reacts violently with water. Make sure that the sample is kept dry at all times and store the powder in a screw top bottle. Dispose of all organic waste in the waste container provided, and not down the drain! The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 79 MODEL ANSWERS TO QUESTIONS Q1. Observe the end of the glass tube and note what happens. A1. A few seconds after adding the water, a bright yellow-orange flame bursts from the end of the glass tube. The flame continues to burn as ethyne passes through the tube and then slowly extinguishes as the production of ethyne in F1 ceases. A black smoke is given off. Q2. Look upwards and around you. Describe what you see in the air. A2. There are black particles of soot floating in the air. Q3. Why is the flame in this experiment referred to as an oxyacetylene flame and not an acetylene flame? A3. The acetylene produced in F1 mixes with oxygen in the air as it escapes from the end of the glass tube. This mixture ignites in the heat from the microburner. Q4. Complete combustion of a hydrocarbon causes the carbon atoms in the hydrocarbon molecule to be oxidised to their highest oxidation state, +4. See the experiment which shows the complete combustion of ethyne. i. What are the colours of the products of the complete combustion of ethyne? ii. What was the colour of one of the products you noticed after observing the oxyacetylene flame? Name this product and list its oxidation state. iii. Why do you think that the product in ii. above was formed? A4. i. ii. iii. Both carbon dioxide and water are colourless. The soot was black. It was carbon. The oxidation state of carbon is 0. The combustion of the ethyne was incomplete, probably because the temperature at which the gas ignited was not high enough for complete combustion. As a result, the carbon atoms in the ethyne molecules (oxidation state -1) were not oxidised to their highest oxidation state but rather to zero as carbon was formed. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 80 ACID-BASE EXTRACTION OF BENZOIC ACID FROM NAPHTHALENE TEACHER'S GUIDE AND MODEL ANSWERS Introduction The following flow chart outlines the procedure. Mixture O OH + Benzoic acid Naphthalene Ether SOLUTION NaOH Remove ether layer Dry and evaporate Ether aqueous O - O Na + Aqueous layer containing Na salt of Benzoic acid. 1) Wash with ether 2) Add HCl Benzoic acid Benzoic acid precipitate O Ether OH Remove ether layer Dry and evaporate Ether Discard aqueous The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 81 1. Chemicals All the chemicals required for this experiment are found in the requirements table preceding the experiment. 2. Apparatus The vials, propettes, microspatula are all components of the RADMASTE Advanced Microchemistry Kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the conventional comboplate can be affected by the organic reagents. The thermometer is used in the combostill kit. 3. Cautions (Information from Biggs, P. The Physical and Theoretical Chemistry Laboratory: Chemical and Other Safety Information. Oxford University http://physchem.ox.ac.uk/MSDS/#MSDS). Benzoic acid is dangerous if swallowed, it is an eye or respiratory irritant and it may cause allergic respiratory or skin reactions in some people. Wear safety glasses and gloves when using it. Naphthalene, is dangerous if swallowed or inhaled. It can irritate the eyes, skin and respiratory system and is a possible carcinogen. Wear safety glasses, a lab coat and gloves when using it. Diethyl ether is extremely flammable and volatile, so keep it way from open flames. It is harmful by inhalation, ingestion and skin adsoption. So work in a fume hood or a well ventilated room or even outside. NaOH is very corrosive, causes severe burns and it may cause serious, permanent harm to the eyes. It is also very dangerous if ingested. It is harmful by skin contact or by inhalation of dust. It is advisable to wear gloves, safety glasses and work in a well ventilated room. Dilute HCl is corrosive. Don't inhale the vapours, or ingest it in any way. The liquid can cause severe damage to skin and eyes. So use gloves and safety glasses. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 82 MODEL ANSWERS Q1. Why do we shake the reaction vial? A1: To get the reagents (which are in the two different layers) to come into contact with each other and so react. Q2. Which layer does the benzoic acid salt go into ? A2. The lower aqueous layer. Q3. Why do we add drying agent to the first layer transferred to the comboplate ? A3. Because there may be a small amount of water in the ether layer. Drying agent reacts with the water but not with the ether thus removing any remaining water from the ether. Q4. What does the word 'decant' mean? In which steps of the experiment, could we have decanted a liquid? A4. To decant means to pour the liquid layer away, leaving the solid contained in it behind. We could have decanted the ether mixture from the drying agent in steps 10 and 16 rather than using a propette. However, the use of a propette is much less messy. Q5. Which is the ether layer - explain why ! A5. In both cases, the top layer is the ether layer because ether is less dense than water. Q6. What is the precipitate formed upon the addition of HCl? A6. Benzoic acid. Q7. Draw out the line drawings of benzoic acid and naphthalene. A7. O OH naphthalene benzoic acid Q8. Write out a balanced chemical equation for the reaction that occurs in step 5 of the procedure. A8. O O - + O Na + HCl OH + NaCl The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 83 Q9. Write out a balanced chemical equation for the reaction that occurs in step 15 of the procedure. A9. O O OH - + NaOH + O Na + H2O Q10. After the liquid has evaporated, what substance do you see in well F1 of the comboplate? And E1? A10. Naphthalene is in well F1 and benzoic acid is in well E1. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 84 SAPONIFICATION TEACHER'S GUIDE AND MODEL ANSWERS Introduction The hydrolysis of an ester (in this case butter) in basic solution to form a carboxylic acid salt (in this case soap) is called saponification, after Latin sapo, "soap". It occurs through nucleophilic acyl substitution. The hydroxide ion adds to the ester carbonyl group to form a tetrahedral intermediate. An alkoxide ion is then lost to give a carboxylic acid which is then deprotonated to give the carboxylic acid salt (which is the soap product). This reaction may be represented by the following equation; O O O + HO - - O C O HO O - + OH Accounts of the production of soap date back more than 5000 years. Several millennia ago, crude mixtures of animal fats and alkaline plant ash were used to generate soaps which lathered and cleaned effectively. The process of making soap hasn't changed much over the years. Most fats and oils are triesters (ie. the ester molecules of three long chain aliphatic carboxylic acids and a single glycerol molecule). carboxylic acid salt (or soap) These carboxylic acid salts are insoluble in an aqueous solution of NaCl. It is often necessary to precipitate carboxylic acid salts formed in the saponification by a process called "salting out" with aqueous sodium chloride. This is because most soaps are insoluble in an aqueous solution of salt. This explains why it is very difficult to obtain a lather when you wash your hands with soap in sea water. The principal ester in butter is an ester of glycerol with butanoic and other acids. When reacted with NaOH it forms sodium butanoate. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 85 Action with hard water Water is called hard when it contains more than 100 parts per million of the salts of such metals as calcium, magnesium, and iron. When soap is dissolved in hard water, it reacts with these metal ions to form an insoluble carboxylic acid salt. Only when all the metal ions have reacted with the soap, is the water "softened", and the remaining soap is able to clean. 1. Chemicals All the chemicals required for the saponification experiment are found in the requirements table preceding the experiment. Ice or (cold water) is required. 2. Apparatus The vials, propettes, microspatula, microburner, microretort stand, are all components of the RADMASTE Advanced Microchemistry kit. The organic comboplate is manufactured from plastic which is resistant to organic solvents and must be used in this experiment, because the comboplate used with aqueous solutions can be affected by the organic reagents. 3. Hints Ice bath: If no ice is readily available, simply pour cold water into a suitable container, eg. the lunch box, and make the water level about 1-2 cm high. Vacuum filtration: Make sure all the lids, the silicone tubing and other connections are tightly sealed. This will ensure more efficient filtration. Try to get as much soap product out of the reaction vial into the funnel as possible with as little water as possible. If not all the soap is retrieved out of the vial after 0.2 ml water, leave it in the vial. It is not important to get every last grain of soap out, since no quantitative analysis is being conducted. Using too much water to wash out the product from the reaction vial and to wash the product means (1) that some of the soap product will dissolve and (2) that the microburner vial will fill up with water and it will have to be emptied and replaced without spilling any soap product out of the funnel. Therefore, guard against using too much water in these steps. When tying the knot in the string, first tie the knot and then cut the ends off. 4. Cautions (Taken from Biggs, P. The Physical and Theoretical Chemistry Laboratory: Chemical and Other Safety Information. Oxford University http://physchem.ox.ac.uk/MSDS/#MSDS). NaOH is very corrosive, causes severe burns and it may cause serious permanent eye damage. It is also very harmful by ingestion. It is harmful by skin contact or by inhalation of dust. It is advisable to wear gloves, safety glasses and work in a well ventilated room. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 86 MODEL ANSWERS TO QUESTIONS Q1. Butter contains esters of glycerol with several different carboxylic acids. We refer below to butyric (butanoic) acid (R = C3) for simplicity, but many of the acids have R= C15-C17. A1. ! Write out the chemical formula and the line drawing for glycerol. ! Write out the chemical formula and line drawing for butyric acid. H3C OH O CH3CH2CH2COOH Propose a chemical structure for the glycerol tributyrate ester. ! H3C O O O H3C O CH3 O O Write out a balanced chemical equation for the following reaction and identify the soap. ! R2 O HO O O + 3 NaOH Na H2O O 3 R1 O + R HO O R3 O = soap HO O The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 87 ! (optional) Draw out the reaction mechanism for the reaction. O O R4 O + HO R3 O - - R4 O OH R3 R3 OH + R4 O - O - H2C R4 = R1 O O O R3 R2 O R4 + O - OH Q2. Name some other household products that contain fats that could be used in this experiment instead of butter. A2. Margarine, olive oil, sunflower oil, bacon grease, peanut butter, vegetable oil - any of these fats could be used in principle even though some of them are saturated and some of them are unsaturated fats. This should not make any difference since the saponification reaction takes place around the -COOR parts of the molecule and does not involve the breaking of C=C unsaturated bonds. Q3. Why is the soap layer the upper layer and the aqueous layer the lower layer ? A3. The organic soap layer is less dense than the aqueous layer, thus it forms the upper layer. Organic molecules generally form liquids and solids that are less dense that water. Q4. What is the colour of the universal indicator paper ? What is the pH ? Explain your observations. pH reading with universal indicator paper Crude Soap Product Commercial Soap Violet, pH ~ 11 light blue, pH ~ 8 Commercial soaps are normally slightly basic (eg. pH = 8). This is because carboxylic acid salts are weak bases and react with the water to form a low concentration of hydroxide ion. The more strongly basic nature of the crude soap, however, could be due the fact that it was produced in a solution of sodium hydroxide, and if it is not thoroughly washed, some sodium hydroxide may still be present. This poses a dilemma. Too much water to wash the soap can dissolve some of it. Too little can cause NaOH impurities to be present in the solid soap. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 88 Q5. What did you observe when you added the soap to the hard water and soft water ? Explain your observations. A5. Description Tap Water Hard Water The solution turns milky because the soap molecules disperse in the water without forming a precipitate. The solution remains clear, but white pieces of solid float on the surface (see explanation below). When soap is placed in hard water, it reacts with the calcium chloride to form calcium butyrate that is insoluble in water. Only when all the calcium ions have reacted with the soap is the water softened and the remaining soap molecules can disperse in the water giving it a milky appearance as in the tap water well. The white solid that floats on top of the hard water is calcium butyrate (also known as scum). Normally precipitates are more dense than water and they sink to the bottom of the container. In this case the precipitate is less dense that water and it floats on top. Q6. Note what you observe when you add salt to the soap solution, and explain it. A6. The solution changes from being milky to being clear but with larger pieces of solid soap floating around. Soap is soluble in water; it forms "micelles" (see question 7) that scatter the light to make the solution look milky. Soap is insoluble in salt water. When you add salt to the soap solution, the soap precipitates out. EXTENSION QUESTIONS Q7. What is the cause of the cleansing properties of soap? (Hint: look up "micelles" in an organic chemistry textbook). A7. Soaps clean because the opposite ends of the carboxylic acid salt are so very different. The sodium end of the molecule is ionic and therefore, hydrophilic (ie. waterloving). The carbon chain end is non-polar and therefore, lipophilic (ie. fat-loving). When soaps are dissolved in water, the long carbon chain ends of the soap molecule cluster together while the sodium hydrophilic parts stick out into the water (see the figure below). A cluster of such soap molecules is called a micelle. When grease or fat is placed in soapy water, they are coated by the non-polar 'tails' in the center of the micelles, thus dispersing the grease or fat molecules. In this way soap is able to clean fatty stains. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 89 Q8. Predict the product of the following reaction: A8. The UNESCO-Associated Centre for Microscience Experiments RADMASTE Centre, University of the Witwatersrand, Johannesburg, South Africa Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za 90 The UNESCO-associated Centre for Microscience Experiments RADMASTE Centre University of the Witwatersrand 19th Floor, University Corner Corner Jorissen and Bertha Street Braamfontein, Johannesburg South Africa Private Bag 3 WITS 2050 Tel: (+) 27 11 717 4802 Fax: (+) 27 11 403 8733 email: [email protected] website: www.microsci.org.za























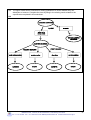














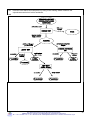



















































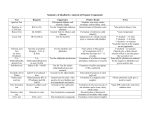





![Group Activity 3 [10 PTS]](http://s1.studyres.com/store/data/010780770_1-3445600a9b56e890a0f283c789afe8fb-150x150.png)


