* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Four Amino Acids Are Converted to Succinyl
Gaseous signaling molecules wikipedia , lookup
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosequestration wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Point mutation wikipedia , lookup
Isotopic labeling wikipedia , lookup
Butyric acid wikipedia , lookup
Microbial metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Catalytic triad wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
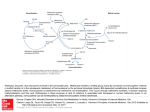
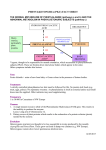
Four Amino Acids Are Converted to SuccinylCoA • The carbon skeletons of methionine, isoleucine, threonine,and valine are degraded by pathways that yield succinyl-CoA, an intermediate of the citric acid cycle. • Only portions of the carbon skeletons are converted to succinylCoA. • Four-fifths of the carbons of valine, three-fifths of those of methionine, and half of those of isoleucine form suuccinyl-CoA. • The carboxyl carbons of of all three form CO2. • The terminal two carbons of isoleucine form acetyl-CoA. • The methyl group of methionine is removed. • Methionine donates its methyl group to one of several possible acceptors through S-adenosylmethionine, and three of its four remaining carbon atoms are converted to the propionate of propionyl-CoA, a precursor of succinyl-CoA. Methionine, isoleucine and valine are converted to succinyl CoA • The 4-C Krebs Cycle intermediate succinyl-CoA is produced from isoleucine, valine, & methionine. • All essential. • Propionyl-CoA, an intermediate on these pathways, is also a product of β-oxidation of fatty acids with an odd number of C atoms. • Methionin could be metabolised to cystein Methionine • Methionine Degradation Requires the Formation of a Key Methyl Donor, S Adenosylmethionine. • IN The first step, L-Methionine condenses with ATP forming Sadenosylmethionine (SAM), active methionine, a common and important methyl donor in the cell. • The activated S-methyl group may transfer to various acceptors. • Removal of methyl group from Sadenosyl homocystein. • Hydrolysis of the S-C bond yields L-homocystein and adenosine. • Homocysteine may be catabolized to cysteine & succinyl-CoA. • Or methionine may be regenerated from homocysteine Methionine H3C H3C S H2 C H2 C H C COO− S H2 C + H2 C H C CH2 COO− N H 3+ Ade ni n e N H 3+ m ethionin e O ATP PPi + Pi H H OH H OH H acceptor THF N 5 -m ethyl-T H F m ethylated acceptor ad enosine H 2 O HS H2 C S -ad en osylm ethionin e (S A M ) H2 C H C COO− H2 C S H2 C H C CH2 COO− N H 3+ Ade ni n e h om ocystein e O N H 3+ H H OH H OH H S -a den osylh om ocystein e • Methionine → S-Adenosylmethionine by ATP-dependent reaction. • methionine may be regenerated from homocysteine by methyl transfer from N5-methyltetrahydrofolate, via a methyltransferase enzyme that uses B12 as prosthetic group. • The methyl group is transferred from THF to B12 to homocysteine. Homocysteine • Homocysteine has two fates. • If there is a deficiency of methionine, homocysteine may be remethylated to methionine. If methionine stores are adequate, homocysteine may enter the transsulfuration pathway, where it is converted to cysteine. • Homocysteine condenses with serine, forming cystathionine, which is hydrolyzed to α-ketobutyrate and cysteine. • This vitamin B6–requiring sequence has the net effect of converting serine to cysteine, and homocysteine to α-ketobutyrate. • α-ketobutyrate is oxidatively decarboxylated to form propionyl CoA by The enzyme α-ketoacid dehydrogenase complex. • Propionyl CoA is processed to succinyl CoA. • Elevations in plasma homocysteine levels promote oxidative damage, inflammation, and endothelial dysfunction, and are an independent risk factor for occlusive vascular disease. Resynthesis of methionine • • Homocysteine accepts a methyl group from N5methyltetrahydrofolate (N5-methyl-THF) in a reaction requiring methylcobalamin, a coenzyme derived from vitamin Bl2. The methyl group is transferred from the B12 derivative to homocysteine, and cobalamin is recharged from N5-methyl-THF. Met, Ile, Thr, Val catabolism • Isoleucine :undergoes transamination, followed by oxidative decarboxylation of the resulting - keto acid. • The remaining five-carbon skeleton is further oxidized to acetyl-CoA(2 carbon) and propionyl-CoA (3 carbon). • Valine: undergoes transamination and decarboxylation, then a series of oxidation reactions that convert the remaining four carbons to propionyl-CoA. • Threonine: is also converted in two steps to propionylCoA. (This is the primary pathway for threonine degradation in humans) Oxidation of propionyl-CoA to Succinyl CoA • The propionyl-CoA derived from these three amino acids is converted to succinyl-CoA by carboxylation to D- methylmalonyl- CoA, epimerization to the L-methylmalonyl-CoA, and conversion to succinyl-CoA by the coenzyme B12–dependent methylmalonyl-CoA mutase reaction. • In the rare genetic disease known as methylmalonic acidemia, methylmalonyl-CoA mutase is lacking with serious metabolic consequences. • Carbon skeletons of ketogenic amino acids are degraded to: acetyl-CoA, or acetoacetate. • Acetyl CoA, & its precursor acetoacetate, cannot yield net production of oxaloacetate, the gluconeogenesis precursor. • Carbon skeletons of ketogenic amino acids can be catabolized for energy in Krebs Cycle, or converted to ketone bodies or fatty acids. • They cannot be converted to glucose. Amino acids that form acetyl CoA or acetoacetyl CoA • • • • • • • • • • • Seven amino acids form acetyl CoA and/or acetoacetyl-CoA. These amino acids are lysine, leucine,tryptophan, phenylalanine, tyrosine,, isoleucine, and threonine. Leucine is ketogenic, forming acetyl CoA and acetoacetate. Lysine is exclusively ketogenic amino acid, converted to acetoacetyl CoA. Tryptophan is both glucogenic and ketogenic because its metabolism yields alanine and acetoacetyl CoA. Isoleucine is both ketogenic and glucogenic, because its metabolism yields acetyl CoA and propionyl CoA. Leucine, isoleucine, lysine, and tryptophan form acetyl CoA or acetoacetyl. Phenylalanine and tyrosine give to acetoacetate during their catabolism. Threonine yields some acetyl-CoA via minor pathway. Leucine, lysine, phenylalanine, tyrosine and tryptophan yield acetyl-CoA via acetoacetyl-CoA. Isoleucine, leucine, threonine, and tryptophan also form acetylCoA directly. Catabolic pathways for tryptophan, lysine, phenylalanine,tyrosine, leucine, and isoleucine • some of their carbons (red) to acetyl-CoA. • Tryptophan, phenylalanine,tyrosine, and isoleucine also contribute carbons (blue) to pyruvate or citric acid cycle intermediates. Tryptophan • Tryptophan breakdownis the most complex of all the pathways of amino acid catabolism in animal tissues; portions of tryptophan(four of its carbons) yield acetylCoA via acetoacetyl-CoA. • Some of the intermediates in tryptophan catabolism are precursors for the synthesis of other biomolecules including nicotinate, a precursor of NAD and NADP in animals; serotonin, a neurotransmitter in vertebrates; and indoleacetate, a growth factor in plants. Phenylalanine • Phenylalanine is hydroxylated to tyrosine. • Phenylalanine and tyrosine have nine carbons . They are degraded into two fragments, both of which can enter the citric acid cycle: four of the nine carbon atoms yield free acetoacetate, which is converted to acetoacetyl-CoA and thus acetylCoA, and a second four-carbon fragment is recovered as fumarate. • Thus eight of the nine carbons of these two amino acids thus enter the citric acid cycle; the remaining carbon is lost as CO2. Phenylalanine → Tyrosine • Phenylalanine is hydroxylated to tyrosine by phenylalanine hydroxylase. • Phenylalanine hydroxylase (also called phenylalanine-4monooxygenase) is one of a general class of enzymes called mixed-function oxidases, all of which catalyze simultaneous hydroxylation of a substrate by an oxygen atom of O2 and reduction of the other oxygen atom to H2O. • Phenylalanine hydroxylase requires the cofactor tetrahydrobiopterin, which carries electrons from NADH to O2 and becomes oxidized to dihydrobiopterin. • It is subsequently reduced by the enzyme dihydrobiopterin reductase in a reaction that requires NADH. Role of tetrahydrobiopterine in phenylalanine hydroxylase reaction Tyrosine • Transamination of tyrosine to p-hydroxyphenylpyruvate is catalyzed by tyrosine α-ketoglutarate transaminase (tyrosine aminotransferase). • P-hydroxyphenylpyruvate forms homogentisate catalysed by phydroxyphenylpyruvate dioxygenase where ascorbic acid is the reductant. • Homogentisate oxidase opens the aromatic ring to form maleylacetoacetate. • Isomerization of maleylacetoacetate-cis, trans isomerase resulting in the formation of fumarylacetoacetate. • Hydrolysis of fumarylacetoacetate forms fumarate and acetoacetate catalysed by fumarylacetoacetate hydrolase. • The acetoacetate forms acetyl CoA plus acetate catalyzed by βketothiolase. Phenylalanine and Tyrosine Metabolism