* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 4
Climate change adaptation wikipedia , lookup
Global warming hiatus wikipedia , lookup
Economics of global warming wikipedia , lookup
Michael E. Mann wikipedia , lookup
Low-carbon economy wikipedia , lookup
Fred Singer wikipedia , lookup
Climate governance wikipedia , lookup
Media coverage of global warming wikipedia , lookup
Climate change and agriculture wikipedia , lookup
Effects of global warming on human health wikipedia , lookup
Mitigation of global warming in Australia wikipedia , lookup
Climate change in Tuvalu wikipedia , lookup
Global warming wikipedia , lookup
Citizens' Climate Lobby wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Scientific opinion on climate change wikipedia , lookup
Politics of global warming wikipedia , lookup
Climatic Research Unit documents wikipedia , lookup
Physical impacts of climate change wikipedia , lookup
Climate sensitivity wikipedia , lookup
Climate engineering wikipedia , lookup
General circulation model wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Climate change feedback wikipedia , lookup
Climate change in the United States wikipedia , lookup
Effects of global warming on humans wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
Climate change, industry and society wikipedia , lookup
Climate change and poverty wikipedia , lookup
Instrumental temperature record wikipedia , lookup
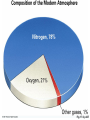
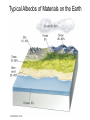
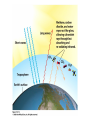
Global Warming Climate • Climate: the average weather conditions over a period of years in a particular place • Climate is influenced by a variety of processes, including geologic processes – Volcanism – Sea-floor spreading – Configuration of landmasses due to plate tectonics • Climate changes impact geologic processes – Rates of erosion and deposition – Types of sediments deposited and sedimentary rocks formed – Geomorphology (surface features) – Fossil record The Climate System • Multidimensional System, many interacting parts • Atmosphere, hydrosphere, geosphere, biosphere, and cryosphere • Exchange of energy and moisture The Climate System Paleoclimatology – the study of past climates. • Earth’s climate varies in cyclical fashion over a number of time-scales • The study of natural climate processes is important to understand the role of humans in climate change. • Scientists measure climate change in the past in many different ways, depending on the timescale. Paleoclimatology – Study of past climates What can paleoclimatology tell us about climate change that is relevant to society in the future? • Is the last century of climate change unprecedented relative to the last 500, 2000, and 20,000 years? • Do recent global temperatures represent new highs, or are they just part of a longer cycle of natural variability? • Is the recent rate of climate change unique to the present or was it commonplace in the past? • Can we find evidence in the paleoclimate record for mechanisms or climate forcings that could be causing recent climate change? Proxy Climate Indicators • Instrumental records (from thermometers, rain gauges, etc.) only exist for the last 150 years. • Proxy climate indicators provide indirect indications of climate change. These include: – – – – – – Seafloor Sediments Oxygen Isotopes Glacial Ice Cores Corals Pollen Historical Data Proxy climate indicators and their useful time range Climate Data from Historical Records Wine is a serious business in Europe! Careful records of the first day of the grape harvest in Europe have been kept since the 14th century. Trends in these records show changes in climate, as the harvest started earlier or later in the year. Tree rings •Trees can live for thousands of years. •The width of tree rings provides information about growing conditions, including temperature conditions and CO2 concentrations. Oxygen Isotope Analysis One of the most important ways that proxy data indicators reveal climate information is through the use of oxygen isotope analysis. Oxygen Isotope Analysis • Isotope – varieties of an element with different numbers of neutrons, resulting in different atomic masses. • The most common isotope of oxygen has an atomic mass of 16 and is called 16O. A heavier, less common variant is 18O. Both occur naturally, and neither is radioactive. You breathe both kinds. Both isotopes bond with 2 hydrogen atoms to make water, H2O. • Water made of 16O evaporates more easily. Water made of 18O condenses more easily. 18O has two extra neutrons Oxygen Isotope Analysis • During colder weather, more light 16O evaporates, leaving ocean water with more heavy 18O. • This oxygen is incorporated into coral, plankton shells and sediments at the ocean bottom. • In colder climates, these proxy indicators will be enriched in 18O. • The ratio of 18O to 16O, (δ 18O) can be correlated to temperature. For benthic (deep-water sediments), colder temperatures are related to higher values of δ 18O. Reading the Graph • Horizontal (x) axis: Note older data as you move right. • Left vertical (y) axis - δ 18O ratios. A value of zero indicates the “standard” value for ocean water. The higher the value, the more 18O, and the colder the ocean waters. Note colder is down on this graph. • Right vertical (y) axis - ∆T (temperature difference from “normal” annual average ocean temperature) Zero (dashed line) indicates “normal”. Oxygen Isotope Analysis •Conversely, glacial ice cores are made of evaporated water that precipated. • Precipitation water always has a negative value of of δ 18O. • Colder temperatures are related to lower (negative values) of δ 18O. •The arrow points to a sudden cooling event that occurred over 10, 000 years ago, known as the Younger Dryas Event. Great website explaining oxygen isotope analysis http://earthobservatory.nasa.gov/Features/P aleoclimatology_OxygenBalance/ Left: The type of fossil can indicate the temperature range in ocean waters. Their shells can be analyzed chemically to measure CO2 concentrations and oxygen isotope ratios Oxygen isotope analysis (below): the ratio of O-18 (heavier, less common) to O-16 (lighter, more common) is a proxy measurement of temperature. Ice Cores – a very valuable proxy data indicator •Ice cores have annual rings, like trees, so age of core can be determined •Air bubbles trapped in the ice can be analyzed for oxygen-isotope data, carbon dioxide concentration, presence of aerosols etc. •The ice itself can be melted and analyzed for these proxy data indicators. Vostok Ice Core Data • • • • This is partial data from an ice core from Antarctica. Temperature data from oxygen/hydrogen isotopes. Interglacial” is a warm period between ice ages. Peak of last ice age about 20,000 years ago. The modern atmosphere • Two most abundant gases: – 78% N2 – 21% O2 • Less abundant gases (< 1%) – Argon – Water vapor – CO2 (only about .035%) It’s up to almost .040% now! • Non-gaseous components – water droplets – dust, pollen, soot and other particulates Fig. 17.6, p.437 Thermal Structure of Atmosphere: Upper Layers • Troposphere - Extends to about 12 km (40,000 ft) elevation. Where we live. • Stratosphere – heated primarily by solar radiation – Ozone (O3) layer absorbs UV energy, causing temperatures to rise – Above 55km (stratopause) temps fall again • Mesosphere – thin air (can’t absorb energy), very cold up to 80km • Thermosphere – above 80km, temps rise rapidly (to just below freezing!) Solar Energy (Insolation) – Also called solar radiation, although NOT radioactive! – Composed of electromagnetic waves with different properties depending on wavelength, frequency • Longwave (low frequency): includes heat (infrared), radio waves • Shortwave (high frequency): includes visible light as well as ultraviolet, x rays, gamma rays • Electromagnetic spectrum – shows EM wavelengths by frequency and wavelength. Electromagnetic Spectrum Solar Radiation in the atmosphere • Reflection/scattering – bounces off with no effect • Absorption – eventual re-emission in a different form Reflection and Albedo • Reflection–electromagnetic radiation bouncing of from a surface without absorption or emission, no change in material or energy wavelength • Albedo – proportional reflectance of a surface – – – – – a perfect mirror has an albedo of 100% Glaciers & snowfields approach 80-90% Clouds – 50-55% Pavement and some buildings – only 10-15% Ocean only 5%! Water absorbs energy. Typical Albedos of Materials on the Earth Absorption and Emission • Absorption of radiation – electrons of absorbing material are “excited” by increase in energy – Increase in temperature; physical/chemical change – Examples: sunburn, cancer • Emission of radiation – excited electrons return to original state; radiation emitted as light or heat • Earth absorbs short wave radiation from sun (i.e. visible light) and emits longwave (infrared or heat) into the atmosphere. “Greenhouse gases” (water vapor, carbon dioxide, methane, etc.) let shortwave energy pass, but absorb longwave energy radiated upward by the Earth. The longwave energy is then re-radiated by the gases in all directions, some of it returning to the Earth’s surface. The greenhouse effect keeps our atmosphere at a livable temperature of about 15 degrees C (59 degrees F). If all heat escaped, the average temperature of Earth would be about -200 C (00 F).