* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lab#6 Prelab CARBOXYLIC ACIDS
Survey
Document related concepts
Genetic code wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Transcript
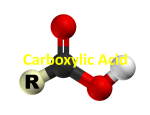
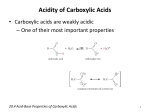
Chemistry 108 Carboxylic Acids Prelab Prelab 6: Carboxylic Acids The Structure of Carboxylic Acids Carboxylic acids contain a carboxyl functional group attached to a hydrocarbon (alkyl group) part. Carboxyl groups contain both a carbonyl group, which is a carbon double bonded to an oxygen (C=O), and a hydroxyl group (-OH) that are connected to each other and the hydrocarbon part as shown below. The carboxyl group is sometimes drawn as “COOH” or “CO2H” in condensed structures: Chemists use the letter “R” in the general structures to represent either a hydrocarbon/alkyl group part or any other organic group of atoms. general form of a carboxylic acid molecule Carboxyl groups are found in amino acids, proteins, fatty acids and oils, and in intermediates of carbohydrate metabolism. Preparation (synthesis) of Carboxylic Acids: Aldehydes can be oxidized to carboxylic acids. The general form of the equation for the oxidation of an aldehyde is shown below. aldehyde carboxylic acid In this experiment, you will oxidize benzaldehyde to form benzoic acid. You will do this reaction twice, in one activity, you will use oxygen gas (O2) from air as the oxidizing agent, and in a second activity you will use permanganate ions (MnO4-) as the oxidizing agent. The R group in benzaldehyde is a benzene ring. In the following problem, I would like you to draw the structural formula of carboxylic acid (benzoic acid) that is produced when benzaldehyde is oxidized. 1 Chemistry 108 Carboxylic Acids Prelab Chemical Reactions of Carboxylic Acids 1) Reaction of Carboxylic Acids with Water Carboxylic acids have the word “acid” in their names because they readily donate an H+ in acid-base reactions. When placed in water, a carboxylic acid molecule acts as an acid and water acts as a base. An H+ from the hydroxyl group (OH) of the carboxylic acid is donated to H2O. The base form of a carboxylic acid is called a carboxylate ion. • A carboxylate ion has a charge of (1-) because of the formal charge on the single-bonded oxygen. Recall that an oxygen atom with just one single bond has a (1-) formal charge. A specific example of the reaction of a carboxylic acid and water is the reaction ethanoic acid and water. The base form of ethanoic acid is named ethanoate ion. Carboxylate ions are named by replacing the “-ic acid” suffix of their acid form name with “-ate ion.” When a carboxylic acid is placed in water, a reaction occurs between the acid and the water, and an equilibrium is established. There is some of the acid form present and some of the base form present at equilibrium. Carboxylic acids are considered to be “weak acids” because their Ka values are much less than 1. When carboxylic acid/carboxylate ion conjugate pairs are in a solution, even if the solution contains other dissolved species, the relative amounts of the acid form and base form can be predicted by the Henderson-Hasselbalch Equation. • The implications of the Henderson-Hasselbalch Equation are shown in the table on the right. 2 Chemistry 108 Carboxylic Acids Prelab 2) Neutralization: Reaction of a Carboxylic Acid and a Hydroxide Ion In a neutralization reaction, a carboxylic acid will react with a hydroxide-containing base compound to produce H2O and a carboxylic acid salt. This is the same neutralization reaction that you learned in a previous chapter; the H+ from the acid bonds to the OH- to produce H2O. The carboxylate ion and the hydroxide from the base make an ionic compound called a carboxylic acid salt. Water Solubility of Carboxylate Ions A carboxylic acid salt formed from a carboxylate anion and a Na+ or K+ cation is water soluble if its “R” group contains less than twelve carbon atoms. If its “R” group contains twelve or more carbons, then it is water insoluble. • The attraction of water to the formal charge of the carboxylate ion makes the salts more water soluble than their carboxylic acid conjugates. A carboxylic acid salt formed from a carboxylate anion and a Ca2+ or Mg2+ cation is water insoluble. 3) Esterification: The Reaction of a Carboxylic Acid and an Alcohol In an esterification reaction, a carboxylic acid reacts with an alcohol to produce an ester and water. The general form for the esterification reaction is: In order to keep track of them in the general reaction, we use “R” for the hydrocarbon part of the carboxylic acid, and “R’ ” for the hydrocarbon part of the alcohol. • R and R’ may, or may not, be identical. An ester is produced when the OH from the carboxylic acid is replace with the OR’ from the alcohol. The OH from the carboxylic acid combines with the H from the alcohol to produce H2O. Esterification reactions can be done in the lab by heating a carboxylic acid and alcohol mixture in the presence of a strong acid catalyst. A specific example of an esterification reaction is the reaction of ethanoic acid and pentanol (an alcohol): 3 Chemistry 108 Carboxylic Acids Prelab The ester formed in the reaction above, pentyl ethanoate, has the distinct aroma of bananas. Many esters have pleasant aromas and flavors, and occur naturally in foods. Esters are often added to foods as artificial flavors. They are also used in perfumes. The Greek delta symbol (∆) is written below the arrows in the chemical equation when heat is used to increase the rate of a reaction, as shown in the equation above. Likewise, when catalysts, such as H3O+, are used, the formula or name of the catalyst is written above the arrows. Naming Esters The IUPAC method for naming esters involves naming the R’ alkyl group part first, followed by the “carboxylate-like” part. Example: Name the ester shown below: SOLUTION: 1) Identify the alkyl group (R’) part and the carboxylate-like part. 2) The ester is named by writing the alkyl group (R’) part name first, then a space, followed by the name that the “carboxylate-like” part would have if it were an actual carboxylate ion. In this example, the “carboxylate-like” part contains three carbons, therefore its name would be propanoate if it were an actual carboxylate ion. The alkyl group (R’) is an ethyl group. The name of this ester is ethyl propanoate. Many naturally-occurring esters contain alkyl groups composed of more than four carbons. In chapter 4, you learned the names of alkyl groups with four or fewer carbons (i.e. methyl, ethyl, propyl, butyl). The table on the right lists the names and structures of nonbranched alkyl groups composed of 5-10 carbons. 4 Chemistry 108 Carboxylic Acids Prelab 4) Decarboxylation of Carboxylic Acids Carboxylic acids undergo a decomposition reaction called decarboxylation. This reaction is very important in the citric acid cycle and other biological processes. • The carbon dioxide that we exhale is produced by decarboxylation reactions in two of the reactions of the citric acid cycle. In decarboxylation reactions, a carboxyl group (COOH) is removed and replaced by a hydrogen atom. The general form for the decarboxylation reaction is: Decarboxylation reactions require heat and/or a catalyst. The enzymes in biological systems that catalyze decarboxylation reactions are called decarboxylases. 5) The Reaction of Carboxylic Acids with Amines An amide is produced when a carboxylic acid reacts with an amide or ammonia (NH3). The general form of this reaction is shown below. An easy way to predict and draw the products of this reaction is to: 1) Draw the structures of the carboxylic acid and the amine (or ammonia) with the (OH) from the carboxylic acid and an H from the amine (or ammonia) adjacent to each other. 2) Remove the (OH) from the carboxylic acid and an H from the amine (or ammonia), and then combine the H and OH to make H2O. 3) Bond the nitrogen and its remaining groups to the carbonyl carbon of the carboxylic acid. An amide can form when a carboxylic acid reacts with ammonia, with a primary, or with a secondary amine. • Examples: a) Formation of an amide by the reaction of a carboxylic acid and ammonia. 5 Chemistry 108 Carboxylic Acids Prelab b) Formation of an amide by the reaction of a carboxylic acid and a 1o amine. The “N-methyl” in “N-methylethanamide” indicates that there is a methyl substituent bonded to the nitrogen. c) Formation of an amide by the reaction of a carboxylic acid and a 2o amine. The “N,N-dimethyl” in “N,N-dimethylethanamide” indicates that there are two methyl substituents bonded to the nitrogen. Amides are not formed from 3o amines because 3o amines do not have a hydrogen attached to the nitrogen. Questions: 1) Name the following carboxylic acids: a) CH3CH2CH2COOH Name: ___________________________ b) CH3CHCH2CH2COOH: | CH3 Name: ___________________________ 2) Draw line bond structures for the following carboxylic acids: a) 2,3-dibromohexanoic acid b) 3-methylpentanoic acid 6 Chemistry 108 Carboxylic Acids Prelab 3) What are the products of the reaction of ethanoic acid reacts with1-butanol (CH3CH2CH2CH2OH). 4) What are the products of the reaction of propanoic acid reacts with ethanamine. 5) What are the products of the reaction of butanoate ion with hydrochloric acid (HCl). 7