* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Towards the Molecular Basis of Sperm and Egg Interaction during
Ligand binding assay wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Paracrine signalling wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Biochemical cascade wikipedia , lookup
Signal transduction wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
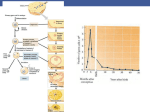
Cells Tissues Organs 2001;168:36–45 Towards the Molecular Basis of Sperm and Egg Interaction during Mammalian Fertilization Paul M. Wassarman Eveline S. Litscher Department of Biochemistry and Molecular Biology, Mount Sinai School of Medicine, New York, N.Y., USA Key Words Sperm W Eggs W Fertilization W Species specificity W mZP3 W Sperm receptor W Acrosome reaction W Oligosaccharides W Mutations Abstract During the past 2 decades, a number of genes have been cloned from mammals which encode polypeptides that participate in the process of fertilization. Among these are glycoproteins ZP1–3 that constitute the zona pellucida of eggs from mice to human beings. In mice, one of these glycoproteins, mZP3, acts as a primary sperm receptor and acrosome reaction-inducer. The evidence suggests that acrosome-intact sperm recognize and bind to a specific class of mZP3 oligosaccharides present on two serine residues (O-linked) located near the carboxyterminus of the polypeptide. Mutagenesis of either of these residues results in the synthesis of an inactive form Abbreviations used in this paper CHO EC hZP3 mZP3 ZP Chinese hamster ovary embryonal carcinoma hamster ZP3 mouse ZP3 zona pellucida ABC Fax + 41 61 306 12 34 E-Mail [email protected] www.karger.com © 2001 S. Karger AG, Basel Accessible online at: www.karger.com/journals/cto of the receptor. Therefore, mammalian fertilization is a carbohydrate-mediated event. It is possible that changes in the structure of these oligosaccharides (e.g., composition, sequence, linkages, modifications, etc.) could account for species-specific binding of sperm to eggs. Stably transfected somatic cells, null mutant animals, and DNA constructs are now available to test this possibility both in vivo and in vitro. Copyright © 2001 S. Karger AG, Basel Introduction How do free-swimming sperm and ovulated eggs from the same species recognize one another and bind to each other? The answer to these questions is of great interest since binding of sperm to eggs initiates a pathway that leads to fertilization of eggs and development of a new individual of the species. An understanding of the molecular basis of this event could have many practical consequences for human beings. For example, it could lead to development of novel methods of contraception [Paterson et al., 2000], as well as to novel therapies that correct infertility. In addition, it could lead to new insights into cellular recognition, adhesion, and signalling in general [Villalobo and Gabius, 1998]. In mammals, fertilization begins with the binding of sperm to the extracellular coat of ovulated eggs, called the Paul M. Wassarman, PhD, Department of Biochemistry and Molecular Biology, Mount Sinai School of Medicine One Gustave L. Levy Place, New York, NY 10029-6574 (USA) Tel. +1 212 241 8616, Fax +1 212 427 7532 E-Mail [email protected] 1 Fig. 1. Light photomicrograph (Nomarski DIC) of mouse sperm bound to the ZP of an ovulated mouse egg in vitro. Fig. 2. Light photomicrograph (dark-field) of mouse sperm, eggs and embryos at the end of an in vitro binding assay. Sperm are bound to the ZP of ovulated eggs, but are not bound to the ZP of two-cell embryos. 2 zona pellucida (ZP) [Gwatkin, 1977; Wassarman, 1987, 1999a; Yanagimachi, 1994; Snell and White, 1996] (fig. 1). All mammalian eggs have a ZP and its thickness varies among different species [Sinowatz et al., 2001]. Although the restrictions on binding imposed by the ZP are not absolute, they provide a relatively high degree of species-specific fertilization in vitro. Notably, removal of the ZP from unfertilized mammalian eggs, thereby exposing egg plasma membrane directly to sperm, eliminates the barrier to interspecific fertilization in vitro for many, but not all mammals [Yanagimachi, 1977, 1981, 1994]. Furthermore, whereas sperm can bind to the ZP of unfertilized eggs, they fail to bind to the ZP of fertilized eggs or cleavage-stage embryos (fig. 2). These observations have led to the idea that the unfertilized egg ZP possesses sperm receptors to which free-swimming sperm of the same species can bind. Following fertilization, the receptors are altered in such a way that binding of sperm is precluded. For more than a decade, considerable evidence has accumulated suggesting that binding of sperm to eggs in mammals is a carbohydrate-mediated event [Wassarman, 1992; Litscher and Wassarman, 1993; Miller and Shur, 1994; Chapman and Barratt, 1996; Benhoff, 1997; Tulsiani et al., 1997; Sinowatz et al., 1998; Töpfer-Petersen, 1999; Wassarman, 1999a, Prasad et al., 2001]. Apparently, sperm recognize and bind to specific oligosaccharides associated with a specific egg-ZP glycoprotein [for pig see also Nakano and Yonezawa, 2001; for human see also Focarelli et al., 2001; Oehninger, 2001]. Therefore, as in the binding of bacteria, animal viruses, and other pathogens to their cellular hosts, binding of pollen to the plant stigma, and sexual agglutination in yeast, binding of sperm to eggs, for both vertebrate and invertebrate species, is mediated by carbohydrates [the basic principles are outlined in Solis et al., 2001]. The variety of oligosaccharide structures potentially available to an organism is staggering [Drickamer and Taylor, 1998], easily providing a molecular basis for species-specific interactions between gametes. Here, we focus on experiments carried out primarily in our own, as well as in other laboratories, which lead to the conclusion that mouse sperm recognize and bind to oligosaccharides covalently linked to mouse egg-ZP glycopro- Mammalian Gamete Interactions Cells Tissues Organs 2001;168:36–45 37 Fig. 3. Electrophoretic separation of mZP1, mZP2 and mZP3. Shown are the positions and molecular weights of radiolabelled mouse ZP glycoproteins following one-dimensional SDS-PAGE under nonreducing conditions and autoradiography. Note the breadth of the individual bands, reflecting heterogeneous N- and O-linked glycosylation of unique polypeptides. tein mZP3. It is proposed that changes in the structure of these oligosaccharides (e.g., their composition, sequence, linkage, and/or modification), together with changes in the egg-binding protein(s) on sperm that recognize mZP3 oligosaccharides, account for species-specific binding of sperm to eggs in mammals [Wassarman and Litscher, 1995; Wassarman, 1999a]. Null mutant mice and appropriate reagents (e.g., DNA and cDNA constructs and promoters) are now available to test this proposal in vivo. Mouse Egg ZP The mouse egg ZP is F6.2 Ìm thick, contains F3.5 ng of protein, and stains positively for carbohydrate (e.g., by PAS or fluorescent lectins) [Wassarman, 1988; Dietl, 1989]. It consists of three, relatively acidic glycoproteins, called mZP1–3, that have apparent molecular weights of F200, F120 and F83 kD, respectively. The glycoproteins appear as broad bands on SDS-PAGE gels as a consequence of heterogeneous glycosylation of a unique polypeptide (fig. 3). The polypeptides of mZP1–3 have apparent molecular weights of F75 kD (dimer of F150 kD held together by intermolecular disulfides), F75 kD (monomer) and F44 kD (monomer), respectively. However, the polypeptides are subjected to proteolytic processing at their amino-terminus (N-terminal ‘signal se- 38 Cells Tissues Organs 2001;168:36–45 quence’) and carboxy-terminus (C-terminal ‘furin cleavage-site’) prior to secretion [Litscher et al., 1999] (fig. 4). The glycoproteins possess both asparagine (N-)-linked (complex-type) and serine/threonine (O-)-linked oligosaccharides. The three ZP glycoproteins are synthesized and secreted exclusively by oocytes during their relatively short growth phase (F2–3 weeks). During this period, the ZP increases in thickness as the oocyte increases in diameter from F12 to F80 Ìm. At about the time of ovulation, synthesis and secretion of ZP glycoproteins cease. The ZP is a very porous extracellular matrix, permeable to large macromolecules (e.g., immunoglobulins) and small viruses [Gwatkin, 1977]. As expected, ZP glycoproteins are organized in a specific manner within the extracellular coat [Greve and Wassarman, 1985; Wassarman and Mortillo, 1991; Green, 1997; Wassarman, 1999a; Sinowatz et al., 2001]. Dimers composed of mZP2 and mZP3 polymerize into long filaments that constitute the ZP. This arrangement of mZP2-mZP3 dimers accounts for a structural periodicity of F140–150 Å seen along ZP filaments in electron micrographs of undecorated and IgG-decorated material (fig. 5). mZP1, a dimer of identical polypeptides, serves as the cross-linker between filaments. The regions of ZP glycoproteins that support these interactions (e.g., between mZP2 and mZP3 and between mZP1 and mZP2 and/or mZP3) have not been determined as yet. mZP1–3 are found in the ZP of eggs from virtually all mammalian species, from mice to human beings [Wassarman, 1999a, b]. In fact, the ZP polypeptides of even the most distantly related species are very similar to each other (i.e., mouse and human ZP3, 170% identical). Furthermore, the vitelline envelope surrounding eggs from fish, amphibians and birds also contains glycoproteins whose polypeptides resemble those of ZP glycoproteins. Thus, there is a significant evolutionary link between glycoproteins of the nonmammalian egg vitelline envelope and glycoproteins of the mammalian egg ZP. It is clear that the site(s) of expression of genes encoding these glycoproteins, growing oocytes, follicle cells and/or liver, varies from organism to organism. A structural role for mZP3 in the ZP is supported by the phenotype of null mutant mice. Targeted disruption of the mZP3 gene by homologous recombination in embryonic stem cells has no effect on the phenotype of male mice, but results in infertility in homozygous null females [Liu et al., 1996; Rankin et al., 1996]. Ovaries from the homozygous null females (mZP3 –/– ) contain growing oocytes that completely lack a ZP, while oocytes from heterozygous null females (mZP3+/– ) have a ZP that is about Wassarman/Litscher Fig. 4. Primary structure and structural landmarks of the mZP3 polypeptide. Shown is the amino acid sequence (single letter amino acid code) of the mZP3 precursor polypeptide (424 amino acids) and the mZP3 mature polypeptide (331 amino acids) after removal of the amino-terminal ‘signal sequence’ (22 amino acids) and carboxy-terminal peptide (71 amino acids) at the ‘furin cleavage-site’. Also indicated are the positions of the cysteine residues (P), potential N-linked glycosylation sites ($), immunoglobulin-like hinge region, the sperm-combining site with O-linked oligosaccharides at serine-332 and serine-334 (black lines), and the hydrophobic region (22 amino acids) of the carboxy-terminal peptide released by proteolytic cleavage. one half the thickness (F2.7 Ìm) of the wild-type [Wassarman et al., 1997]. These results are consistent with the proposed structural role for mZP3, as well as with current models for ZP structure [Wassarman and Mortillo, 1991; Wassarman et al., 1996]. Furthermore, they are consistent with results of experiments in which mZP2 and mZP3 messenger RNA was degraded in the presence of a large excess of complementary (antisense) oligonucleotide injected into the cytoplasm of isolated growing mouse oocytes [Tong et al., 1995]. It was found that the absence of synthesis of either glycoprotein prevented the incorporation of the other glycoprotein into the ZP. It should be noted that a ZP can be restored to oocytes and eggs from mZP3 –/– mice by inclusion of a wild-type ZP3 gene (in this case, encoding human ZP3) as a transgene in these animals [Rankin et al., 1998]. Mouse ZP3 Is a Primary Sperm Receptor Only acrosome-intact sperm bind to the ovulated mouse egg ZP. Experimental evidence strongly supports the conclusion that, during binding of sperm to eggs, mZP3 serves as a receptor for sperm. For example, of the three glycoproteins that constitute the ZP, only purified mZP3 binds exclusively to heads of acrosome-intact Mammalian Gamete Interactions Fig. 5. Transmission electron photomicrograph of solubilized mouse ZP filaments. Shown is an enzyme-solubilized ZP preparation adsorbed to substrate-coated grids and negatively stained. Note the structural repeat located every 140–150 Å or so along the filaments, as well as the interconnections between the long filaments. Cells Tissues Organs 2001;168:36–45 39 Fig. 6. In vitro assays of egg mZP3 and embryo mZP3 sperm recep- tor activity. Binding of mouse sperm to eggs was examined in the absence of mZP3 (hatched circle) and in the presence of either purified egg mZP3 ([) or two-cell embryo mZP3 (P) at several concentrations. Note that egg mZP3 is a very effective inhibitor at nanomolar concentrations, whereas embryo mZP3 is inactive as an inhibitor. sperm and thereby prevents sperm from binding to ovulated eggs in vitro [Bleil and Wassarman, 1980, 1986; Wassarman, 1990; Mortillo and Wassarman, 1991; Wassarman and Litscher, 1995]. Even at nanomolar concentrations, purified, unfertilized egg mZP3 is a very effective inhibitor of sperm binding in this competition assay (fig. 6). On the other hand, at similar concentrations, mZP3 from fertilized eggs or early embryos has no effect on binding of sperm to eggs in vitro. This is consistent with the failure of free-swimming sperm to bind to the ZP of fertilized eggs and preimplantation embryos. It can be concluded from these and other observations [e.g., Bleil and Wassarman, 1980; Florman and Wassarman, 1985; Wassarman, 1990] that, as a consequence of the zona reaction, mZP3 is altered such that free-swimming sperm can no longer recognize and bind to the glycoprotein. Interestingly, the ability of mZP3 to act as a sperm receptor in vitro is not significantly affected by high temperatures, detergents, denaturants, reducing agents, or limited proteolysis. Apparently, mZP3 bioactivity is not dependent on the extent of glycosylation of its polypeptide or on sulfation and sialylation of its oligosaccharides [Liu et al., 1997]. Even after extensive proteolysis of mZP3, the small glycopeptides produced retain activity as a sperm receptor, although higher than normal concentrations are required [Florman et al., 1984; Florman and 40 Cells Tissues Organs 2001;168:36–45 Fig. 7. In vitro assays of binding of sperm to eggs in the presence of tetraantennary octadecasaccharides. Shown are the structures of the two oligosaccharides, one terminating in Gal·1 and the other in GlcNAcß1 (nonreducing terminus), used as potential inhibitors of binding of mouse sperm to eggs. The two oligosaccharides were assayed at three different concentrations, from 1 to 10 ÌM. Note that the oligosaccharide terminating in Gal·1 was a potent inhibitor, whereas the oligosaccharide terminating in GlcNAcß1 caused only background levels of inhibition [Litscher et al., 1995]. Wassarman, 1985]. These observations suggest that mZP3 polypeptide does not play a direct role in sperm receptor function. However, there is considerable information to suggest that mZP3 oligosaccharides play a direct role in sperm receptor function. For example, chemical or enzymatic removal of all mZP3 oligosaccharides results in complete inactivation of the glycoprotein as a sperm receptor. Furthermore, O-linked oligosaccharides recovered from mZP3 by mild alkaline hydrolysis under reducing conditions [Florman and Wassarman, 1985; Bleil and Wassarman, 1988; Miller et al., 1992] and certain O-linked related oligosaccharides [Litscher et al., 1995; Johnston et al., 1998] inhibit binding of sperm to eggs in vitro at micromolar concentrations (fig. 7). Results of some of these studies suggest that galactose, N-acetylglucosamine and/or fucose are essential sugars for sperm Wassarman/Litscher Fig. 8. Site-directed mutagenesis of the mZP3 gene at its sperm-combining-site. a Schematic diagram of the PGK/mZP3 recombinant gene used to generate stably transfected EC-mZP3 cell lines. pPGK-1 represents the mouse phosphoglycerate kinase1 promoter region. Restriction enzymes ClaI and SstII were used to generate linearized DNA fragments for electroporation of EC (F9) cells. Arrow indicates the transcriptional start site on the PGK-1 promoter. The sites of mutagenesis in exon 7 of the mZP3 gene are indicated as amino acids 329–334. In each case, the amino acid was changed to Ala, Val or Gly (i.e., a nonhydroxyl amino acid). b Summary of site-directed mutagenesis of EC-mZP3. Shown are the amino acid changes caused by mutations in the region of the mZP3 gene encoding amino acids 329– 334. Note that only mutations involving serine-332 and serine-334 resulted in synthesis of an inactive form of the sperm receptor. binding. Collectively, these observations suggest that species-specific binding of sperm to eggs in mammals is a carbohydrate-mediated event. On the other hand, the identity of sugars on mZP3 recognized by sperm remains an unresolved and controversial issue. To locate essential O-linked oligosaccharides on mZP3 polypeptide we utilized limited proteolysis [Rosiere and Wassarman, 1992; Litscher and Wassarman, 1996a], exon swapping [Kinloch et al., 1995] and site-directed mutagenesis [Kinloch et al., 1995; Chen et al., 1998]. Results of such studies suggest that all sperm receptor activity of mZP3 is associated with the carboxy-terminal half of the polypeptide and that the essential oligosaccharides are present on just two of five serine residues, serine- 332 and serine-334, in a region of polypeptide near the carboxy-terminus, a region encoded by exon-7 of the mZP3 gene (8 exons). For example, mutation of either serine-332 or serine-334 to a small aliphatic amino acid results in production of an inactive form of mZP3 in stably transfected cells (fig. 8). Interestingly, of the five serine residues, only these two are conserved from mouse to human ZP3. In this context, the numerous amino acid changes neighboring serine-332 and serine-334 that have occurred during evolution may impose changes in the structure of O-linked oligosaccharides added to ZP3 and, thereby, affect species specificity of sperm-egg interaction [Wassarman and Litscher, 1995]. Mammalian Gamete Interactions Cells Tissues Organs 2001;168:36–45 41 Fig. 9. In vitro assays of mouse egg mZP3, hamster egg hZP3, EC- hZP3, and CHO-hZP3 sperm receptor activity. Shown is the percentage inhibition of sperm binding for mouse (m) and hamster (h) eggs (first letter) and sperm (second letter). Mouse and hamster sperm were exposed to F100 nM egg mZP3, egg hZP3 or recombinant hZP3 (secreted by transfected EC or CHO cells and purified), sperm were incubated with mouse or hamster eggs, and the number of sperm bound per egg determined. Note that EC-hZP3 and CHOhZP3 are only effective inhibitors of binding of hamster sperm to mouse and hamster eggs; they do not inhibit binding of mouse sperm to mouse eggs. Mouse ZP3 Is an Acrosome Reaction Inducer The acrosome is a large secretory vesicle that overlies the nucleus in the apical region of the sperm head [Yanagimachi, 1994; Wassarman, 1999a]. Acrosomal membrane just underlying the plasma membrane is referred to as ‘outer’ acrosomal membrane and that overlying the nucleus is referred to as ‘inner’ acrosomal membrane. Morphologically, the acrosome reaction is seen as multiple fusions between outer acrosomal membrane and plasma membrane at the anterior region of sperm head, extensive formation of hybrid membrane vesicles, and exposure of inner acrosomal membrane and acrosomal contents. Only acrosome-reacted sperm can penetrate the ZP and fuse with egg plasma membrane. It is known that there are many different inducers of the acrosome reaction [Yanagimachi, 1994]. However, it is now generally accepted that ZP3 is the natural agonist that initiates the acrosome reaction upon binding of acrosome-intact sperm to the ZP [Bleil and Wassarman, 1983; Wassarman, 1995; Darszon et al., 1996; Wassarman and Florman, 1997; Florman et al., 1998; Nixon et al., 2001]. Criteria now are available that permit one to distinguish between the, so-called, ‘spontaneous’ acrosome reaction 42 Cells Tissues Organs 2001;168:36–45 and the ZP3-induced acrosome reaction (e.g., sensitivity to pertussis toxin). While purified mZP3 and large mZP3 glycopeptides induce sperm to acrosome-react in vitro, small mZP3 glycopeptides and purified mZP3 O-linked oligosaccharides bind to sperm and inhibit their binding to eggs, but do not induce the acrosome reaction [Wassarman, 1988, 1990]. In the latter context, it has been reported that IgG cross-linking of small mZP3 glycopeptides bound to sperm can induce the sperm to acrosomereact [Leyton and Saling, 1989] and that aggregation of ß-1,4-galactosyltransferase on the sperm surface by mZP3 leads to activation of a G protein complex [Gong et al., 1995]. These findings suggest that induction of the acrosome reaction by ZP3 probably will turn out to be dependent on multivalent interactions at the sperm surface. Mouse and Hamster ZP3 Expressed in Transfected Cells It is clear that mouse sperm bind to hamster eggs and hamster sperm bind to mouse eggs [Gwatkin, 1977; Schmell and Gulyas, 1980; Cherr et al., 1986; Moller et al., 1990]. Consequently, it was not surprising to find that mouse ZP3 (mZP3; F83 kD Mr) binds to hamster sperm and that hamster ZP3 (hZP3; F56 kD Mr) binds to mouse sperm [Moller et al., 1990]. Although the molecular weights of the mature glycoproteins are significantly different from each other, their polypeptides are very similar to each other [F83%; Kinloch et al., 1990]. These observations suggest that the oligosaccharides on mZP3 and hZP3 must have some common structural features that are recognized by mouse and hamster sperm. In view of the above, genomic clones of mZP3 [Kinloch et al., 1988] and hZP3 [Kinloch et al., 1990] were placed under control of the phosphoglycerate kinase-1 (pgk-1) promoter and were expressed in several lines of stably transfected embryonal carcinoma (EC) and Chinese hamster ovary (CHO) cells [Kinloch et al., 1991; Litscher and Wassarman, 1996b]. These cells synthesized and secreted relatively large amounts of recombinant ZP3 into the culture medium. Sperm receptor assays of the purified glycoproteins revealed some unexpected results. Although EC-hZP3 and CHO-hZP3 were receptors for hamster sperm, unlike egg hZP3, they were not receptors for mouse sperm (fig. 9). Binding of EC-hZP3 and CHOhZP3 was completely restricted to hamster sperm. The surprising behavior of recombinant EC-hZP3 and CHO-hZP3 is of interest for several reasons. For example, it supports the conclusion that ZP3 oligosaccharides, rath- Wassarman/Litscher er than polypeptide, are recognized by free-swimming sperm when they bind to the ZP of ovulated eggs. The hZP3 polypeptide synthesized by EC and CHO cells is identical to that synthesized by oocytes of mice carrying hZP3 as a transgene [Kinloch et al., 1992]. Yet EC-hZP3 and CHO-hZP3 are bioactive with hamster, but not mouse sperm, whereas hZP3 synthesized by transgenic mice, like hamster egg hZP3, is active with mouse sperm. It also points out once again that two very similar polypeptides can be differentially glycosylated by transfected cells. These results suggest that addition of O-linked oligosaccharides to nascent hZP3 is not determined by polypeptide structure alone. In this context, although it has been demonstrated that the rate of addition of N-acetylgalactosamine to nascent glycoproteins is influenced by the sequence in which glycosylated serine and threonine residues are located [Elhammer et al., 1993; Nehrke et al., 1996], as yet no consensus sequence for O-linked glycosylation has been reported [Wilson et al., 1991; Gooley and Williams, 1994]. A likely conclusion is that the kinds and distribution of glycosyltransferases present in the cell greatly influence the structure of O-linked oligosaccharides attached to ZP3. This, in turn, could affect the bioactivity of the glycoprotein. Final Comments There is great diversity when it comes to the mechanism of species-specific fertilization. For example, some nonmammalian eggs lack an extracellular coat (e.g., nematode eggs) while others have a vitelline envelope and a jelly coat (e.g., echinoderm and amphibian eggs). In some species sperm enter eggs at a particular site (micropyle) without the need for an acrosome reaction (e.g., nematodes), whereas in others sperm undergo the acrosome reaction upon binding to the jelly coat and then bind in a species-specific manner to the vitelline envelope (e.g., echinoderms and amphibians) [see also Focarelli et al., 2001]. In the latter context, one might think of the ZP, which is unique to all mammalian eggs, as an amalgamation of the jelly coat and vitelline envelope of some nonmammalian eggs. The ZP is the site of both species-specific sperm receptors and an inducer of the acrosome reaction. Here, we related what we have learned about the functions of ZP glycoprotein mZP3 during fertilization, with special emphasis on the role of oligosaccharides in mZP3 function. For example, (1) mZP3 is a structural glycoprotein absolutely required for ZP assembly during oogene- Mammalian Gamete Interactions sis, (2) mZP3 is a sperm receptor that supports binding of free-swimming sperm to unfertilized eggs, and (3) mZP3 is an acrosome reaction inducer for sperm bound to the ZP of unfertilized eggs. At least the sperm receptor and acrosome reaction-inducing activities of mZP3 depend on the glycoprotein’s oligosaccharides. In mice, these oligosaccharides apparently are located on two serine residues located near the carboxy-terminus of the mature polypeptide (fig. 4). This portion of the polypeptide has undergone significant changes during evolution and it is possible that these changes result in alternative oligosaccharide structures for ZP3 from different mammalian species. The cell’s rules for O-linked glycosylation of nascent proteins will have to be determined in order to answer key questions about ZP3 oligosaccharides. Although we know a great deal about mZP3, many more questions remain. A pressing issue concerns the nature of the protein associated with plasma membrane surrounding the sperm head that recognizes and binds to mZP3 oligosaccharides (so-called, egg-binding protein). This area of research, while extremely productive, to date is inconclusive [Wassarman, 1999a; Jansen et al., 2001; Nixon et al., 2001]. A second issue concerns the nature of the ZP3 oligosaccharides that are recognized by egg-binding proteins. We know very little about the structure of these oligosaccharides (e.g., composition, sequence and linkages) or their sites on ZP3 polypeptides from different species of mammalian eggs [Nakano and Yonezawa, 2001; Oehninger, 2001]. Can these oligosaccharides account for the species-specific binding of sperm that is observed experimentally? Finally, it remains to work out the mechanism for inactivation of mZP3 as a sperm receptor following fertilization. Preliminary evidence suggests that inactivation can be attributed to enzymatic destruction of essential mZP3 oligosaccharides (unpubl. results). We hope that answers to these and other questions will be forthcoming and that such progress will be of some benefit to clinicians involved in the manipulation of human reproduction. Acknowledgment Many important contributions have been made by past and present members of our laboratory to research on mammalian fertilization. We are very grateful to all of them. Over the years, much of the research was supported by the National Institute of Child Health and Human Development (currently by HD35105) and by Hoffmann-La Roche, Inc. Cells Tissues Organs 2001;168:36–45 43 References Benoff, S. (1997) Carbohydrates and fertilization: An overview. Mol Hum Reprod 3: 599–637. Bleil, J.D., P.M. Wassarman (1980) Mammalian sperm-egg interaction: Identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20: 873– 882. Bleil, J.D., P.M. Wassarman (1983) Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol 95: 317– 324. Bleil, J.D., P.M. Wassarman (1986) Autoradiographic visualization of the mouse egg’s sperm receptor bound to sperm. J Cell Biol 102: 1363–1371. Bleil, J.D., P.M. Wassarman (1988) Galactose at the non-reducing terminus of O-linked oligosaccharides of mouse egg zona pellucida glycoprotein ZP3 is essential for the glycoprotein’s sperm receptor activity. Proc Natl Acad Sci USA 85: 6778–6782. Chapman, N.R., C.L.R. Barratt (1996) The role of carbohydrate in sperm-ZP3 adhesion. Mol Hum Reprod 2: 767–774. Chen, J., E.S. Litscher, P.M. Wassarman (1998) Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc Natl Acad Sci USA 95: 6193– 6197. Cherr, G.N., H. Lambert, S. Meizel, D.F. Katz (1986) In vitro studies of the golden hamster sperm acrosome reaction: Completion on the zona pellucida and induction by homologous soluble zonae pellucidae. Dev Biol 114: 119– 131. Darszon, A., A. Liévano, C. Beltran (1996) Ion channels: Key elements in gamete signaling. Curr Top Dev Biol 3: 117–167. Dietl, J. (1989) The Mammalian Egg Coat: Structure and Function. Berlin, Springer. Drickamer, K., M.E. Taylor (1998) Evolving views of protein glycosylation. Trends Biochem Sci 2: 321–324. Elhammer, A.P., R.A. Poorman, E. Brown, L.L. Maggiora, J.G. Hoogerheide, F.J. Kézdy (1993) The specificity of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase as inferred from a database of in vivo substrates and from the in vitro glycosylation of proteins and peptides. J Biol Chem 268: 10029–10038. Florman, H.M., C. Arnoult, I.G. Kazam, C. Li, C.M.B. O’Toole (1998) A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: A tale of two channels. Biol Reprod 59: 12–16. Florman, H.M., K.D. Bechtol, P.M. Wassarman (1984) Enzymatic dissection of the functions of the mouse egg’s receptor for sperm. Dev Biol 106: 243–255. Florman, H.M., P.M. Wassarman (1985) O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 4: 313–324. 44 Focarelli, R., G.B. La Sala, M. Balasini, F. Rosati (2001) Carbohydrate-mediated sperm-egg interaction and species-specificity: A clue from the Unio elongatus model. Cells Tissues Organs 168: 76–81. Gong, X.H., D.H. Dubois, D.J. Miller, B.D. Shur (1995) Activation of a G protein complex by aggregation of ß-1,4-galactosyltransferase on the surface of sperm. Science 26: 1718–1721. Gooley, A.A., K.L. Williams (1994) Towards characterizing O-glycans: The relative merits of in vivo and in vitro approaches seeking peptide motifs specifying O-glycosylation sites. Glycobiology 4: 413–417. Green, D.P.L. (1997) Three-dimensional structure of the zona pellucida. Rev Reprod 2: 147–156. Greve, J.M., P.M. Wassarman (1985) Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol 181: 253–264. Gwatkin R.B.L. (1977) Fertilization Mechanisms in Man and Mammals. New York, Plenum Press. Jansen, S., M. Ekhlasi-Hundrieser, E. Töpfer-Petersen (2001) Sperm adhesion molecules: Structure and function. Cells Tissues Organs 168: 82–92. Johnston, D.S., W.W. Wright, J.H. Shaper, C.H. Hokke, D.H. Van den Eijnden, D.H. Joziasse (1998) Murine sperm-zona binding, a fucosyl residue is required for a high affinity spermbinding ligand. J Biol Chem 273: 1888–1895. Kinloch, R.A., S. Mortillo, C.L. Stewart, P.M. Wassarman (1991) Embryonal carcinoma cells transfected with ZP3 genes differentially glycosylate similar polypeptides and secrete active mouse sperm receptor. J Cell Biol 115: 655– 664. Kinloch, R.A., S. Mortillo, P.M. Wassarman (1992) Transgenic mouse eggs with functional hamster sperm receptors in their zona pellucida. Development 115: 937–946. Kinloch, R.A., R.J. Roller, C. Fimiani, D.A. Wassarman, P.M. Wassarman (1988) Primary structure of the mouse sperm receptor’s polypeptide chain determined by genomic cloning. Proc Natl Acad Sci USA 85: 6409–6413. Kinloch, R.A., B. Ruiz-Seiler, P.M. Wassarman (1990) Genomic organization and polypeptide primary structure of zona pellucida glycoprotein hZP3, the hamster sperm receptor. Dev Biol 142: 414–421. Kinloch, R.M., Y. Sakai, P.M. Wassarman (1995) Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc Natl Acad Sci USA 92: 263– 267. Leyton, L., P. Saling (1989) Evidence that aggregation of mouse sperm receptors by ZP3 triggers the acrosome reaction. J Cell Biol 108: 2163– 2168. Litscher, E.S., K. Juntunen, A. Seppo, L. Penttilä, R. Niemelä, O. Renkonen, P.M. Wassarman (1995) Oligosaccharide constructs with defined structures that inhibit binding of mouse sperm to unfertilized eggs in vitro. Biochemistry 34: 4662–4669. Cells Tissues Organs 2001;168:36–45 Litscher, E.S., H. Qi, P.M. Wassarman (1999) Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry 38: 12280–12287. Litscher, E.S., P.M. Wassarman (1993) Carbohydrate-mediated adhesion of eggs and sperm during mammalian fertilization. Trends Glycosci Glycotechnol 5: 369–388. Litscher, E.S., P.M. Wassarman (1996a) Characterization of a mouse ZP3-derived glycopeptide, gp55, that exhibits sperm receptor and acrosome reaction-inducing activity in vitro. Biochemistry 35: 3980–3985. Litscher, E.S., P.M. Wassarman (1996b) Recombinant hamster sperm receptors that exhibit species-specific binding to sperm. Zygote 4: 229– 236. Liu, C., E.S. Litscher, S. Mortillo, Y. Sakai, R.A. Kinloch, C.L. Stewart, P.M. Wassarman (1996) Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci USA 93: 5431–5436. Liu, C., E.S. Litscher, P.M. Wassarman (1997) Zona pellucida glycoprotein mZP3 bioactivity is not dependent on the extent of glycosylation of its polypeptide or on sulfation and sialylation of its oligosaccharides. J Cell Sci 110: 745– 752. Miller, D.J., M.B. Macek, B.D. Shur (1992) Complementarity between sperm surface ß1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature 35: 589–593. Miller, D.J., B.D. Shur (1994) Molecular basis of fertilization in the mouse. Semin Dev Biol. 5: 255–264. Moller, C.C., J.D. Bleil, R.A. Kinloch, P.M. Wassarman (1990) Structural and functional relationships between mouse and hamster zona pellucida glycoproteins. Dev Biol 137: 276– 286. Mortillo, S., P.M. Wassarman (1991) Differential binding of gold-labeled zona pellucida glycoproteins mZP2 and mZP3 to mouse sperm membrane compartments. Development 113: 141–151. Nakano, M., N. Yonezawa (2001) Localization of sperm-ligand carbohydrate chains in pig zona pellucida glycoproteins. Cells Tissues Organs 168: 65–75. Nehrke, K., F.K. Hagen, L.A. Tabak (1996) Charge distribution of flanking amino acids influences O-glycan acquisition in vivo. J Biol Chem 271: 7061–7065. Nixon, B., L. Qingxian, M.J. Wassler, C.I. Foote, M.A, Ensslin, B.D. Shur (2001) Galactosyltransferase function during mammalian fertilization. Cells Tissues Organs 168: 46–57. Oehninger, S. (2001) Molecular basis of human sperm-zona pellucida interaction. Cells Tissues Organs 168: 58–64. Paterson, M., Z.A. Jennings, M. van Duin, R.J. Aitken (2000) Immunocontraception with zona pellucida proteins. Cells Tissues Organs 166: 228–232. Wassarman/Litscher Prasad, S.V., S.M. Skinner, C. Carino, N. Wang, J. Cartwright, B.S. Dunbar (2000) Structure and function of the proteins of the mammalian zona pellucida. Cells Tissues Organs 166: 220– 227. Rankin, T., M. Familiari, E. Lee, A. Ginsburg, N. Dwyer, J. Blanchette-Mackie, J. Drago, H. Westphal, J. Dean (1996) Mice homozygous for an insertional mutation in the ZP3 gene lack a zona pellucida and are infertile. Development 122: 2903–2910. Rankin, T.L., Z.-B. Tong, P.E. Castle, E. Lee, R. Gore-Langton, L.M. Nelson, J. Dean (1998) Human ZP3 restores fertility in ZP3 null mice without affecting order-specific sperm binding. Development 125: 2415–2424. Rosiere, T.K., P.M. Wassarman (1992) Identification of a region of mouse zona pellucida glycoprotein mZP3 that possesses sperm receptor activity. Dev Biol 154: 309–317. Schmell, E.D., B.J. Gulyas (1980) Mammalian sperm-egg recognition and binding in vitro. I. Specificity of sperm interaction with live and fixed eggs in homologous and heterologous insemination of hamster, mouse, and guinea pig oocytes. Biol Reprod 23: 1075–1085. Sinowatz, F., S. Kölle, E. Töpfer-Petersen (2001) Biosynthesis and expression of zona pellucida glycoproteins in mammals. Cells Tissues Organs 168: 24–35. Sinowatz, F., J. Plendl, S. Kölle (1998) Protein-carbohydrate interactions during fertilization. Acta Anat 161: 196–205. Snell, W.J., J.M. White (1996) The molecules of mammalian fertilization. Cell 85: 629–637. Solis, D., J. Jiménez-Barbero, H. Kaltner, A. Romero, H.-C. Siebert, C.-W. von der Lieth, H.-J. Gabius (2001) Towards defining the role of glycans as hardware in information storage and transfer: Basic principles, experimental approaches and recent progress. Cell Tissues Organs 168: 5–23. Mammalian Gamete Interactions Tong, Z.-B., L.M. Nelson, J. Dean (1995) Inhibition of zona pellucida gene expression by antisense oligonucleotides injected into mouse oocytes. J Biol Chem 270: 849–853. Töpfer-Petersen, E. (1999) Carbohydrate-based interactions on the route of a spermatozoon to fertilization. Hum Reprod Update 5: 314– 329. Tulsiani, D.R.P., H. Yoshida-Komiya, Y. Araki (1997) Mammalian fertilization: A carbohydrate-mediated event. Biol Reprod 57: 487– 494. Villalobo, A., H.-J. Gabius (1998) Signaling pathways for transduction of the initial message of the glycocode into cellular response. Acta Anat 161: 110–120. Wassarman, P.M. (1987) The biology and chemistry of fertilization. Science 235: 553–560. Wassarman, P.M. (1988) Zona pellucida glycoproteins. Annu Rev Biochem 57: 415–442. Wassarman, P.M. (1990) Profile of a mammalian sperm receptor. Development 108: 1–17. Wassarman, P.M. (1992) Cell surface carbohydrates and mammalian fertilization; in Fukuda, M. (ed): Cell Surface Carbohydrates and Cell Development. Boca Raton, CRC Press, pp 215–238. Wassarman, P.M. (1995) Towards molecular mechanisms for gamete adhesion and fusion during mammalian fertilization. Curr Opin Cell Biol 7: 658–664. Wassarman, P.M. (1999a) Mammalian fertilization: Molecular aspects of gamete adhesion, exocytosis, and fusion. Cell 96: 175–183. Wassarman, P.M. (1999b) Egg zona pellucida glycoproteins; in Kreis, T., R. Vale (eds): Guidebook to the Extracellular Matrix and Adhesion Proteins. Oxford, Oxford University Press, pp 411–414. Wassarman, P.M., H.M. Florman (1997) Cellular mechanisms during mammalian fertilization; in Hoffman, J.F., J.D. Jamieson (eds): Handbook of Physiology, section 14: Cell Physiology. Oxford, Oxford University Press, pp 885– 938. Wassarman, P.M., E.S. Litscher (1995) Sperm-egg recognition mechanisms in mammals. Curr Top Dev Biol 30: 1–19. Wassarman, P.M., C. Liu, E.S. Litscher (1996) Constructing the mammalian egg zona pellucida: Some new pieces of an old puzzle. J Cell Sci 109: 2001–2004. Wassarman, P.M., S. Mortillo (1991) Structure of the mouse egg extracellular coat, the zona pellucida. Int Rev Cytol 130: 85–109. Wassarman, P.M., H. Qi, E.S. Litscher (1997) Mutant female mice carrying a single mZP3 allele produce eggs with a thin zona pellucida, but reproduce normally. Proc R Soc Lond B 26: 323–328. Wilson, I.B.H., Y. Gavel, G. von Heijne (1991) Amino acid distributions around O-linked glycosylation sites. Biochem J 275: 529–534. Yanagimachi, R. (1977) Specificity of sperm-egg interaction; in Edidin, M., M.H. Johnson (eds): Immunobiology of Gametes. London, Cambridge University Press, pp 255–295. Yanagimachi, R. (1981) Mechanisms of fertilization in mammals; in Mastroianni, L., J.D. Biggers (eds): Fertilization and Embryonic Development in vitro. New York, Plenum Press, pp 82–184. Yanagimachi, R. (1994) Mammalian fertilization; in Knobil, E., J.D. Neill (eds): The Physiology of Reproduction. New York, Raven Press, pp 189–317. Cells Tissues Organs 2001;168:36–45 45