* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download functional differentiation of enterocytes in the follicle
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Magnesium transporter wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
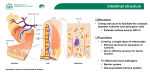
J. Cell Sri. 55, 147-156 (1982) 147 Printed in Great Britain © Company of Biologists Limited 1982 FUNCTIONAL DIFFERENTIATION OF ENTEROCYTES IN THE FOLLICLE-ASSOCIATED EPITHELIUM OF RAT PEYER'S PATCH MICHAEL W. SMITH 1 AND GABRIELLE SYME1 1 Agricultural Research Council Institute of Animal Physiology, Babraham, Cambridge CBz \AT, U.K. 1 Department of Physiology, The University, Sheffield S10 zTN, U.K. SUMMARY The ability of the follicle-associated epithelium (FAE) of rat Peyer's patch to accumulate valine has been measured using a new technique of autoradiographic analysis. Maximum uptake of valine was achieved by enterocytes present in the dome region of the FAE. Valine uptake was not seen in enterocytes present on the lower slopes of the FAE or in follicleassociated crypts. The ability of the FAE to absorb valine was generally much less than that seen in enterocytes present on adjacent villi. The main reason for this discrepancy lay in the apparent inability of the FAE to develop a second phase of amino acid transport similar to that seen in villus enterocytes. It is suggested that this failure results from some unexplained interaction taking place between the FAE and its underlying lymphoid tissue. INTRODUCTION Enterocyte differentiation in the intestinal mucosa is a well characterized phenomenon. Cells born in the crypts of Lieberkuhn rapidly acquire the morphological features of Paneth, goblet, endocrine or columnar absorptive cells, and the final functional differentiation of the absorptive cells takes place as they migrate along the upper regions of individual villi (Kinter & Wilson, 1965). Recently it has been shown that the epithelium covering lymphoid follicles in the intestine (the follicleassociated epithelium, or FAE, of intestinal Peyer's patches) is renewed in the same way and with a time course similar to that determined for surrounding villi (Smith, Jarvis & King, 1980). There are, nevertheless, significant differences between the patterns of differentiation seen in these two tissues, the most obvious being the presence of antigen-transporting cells in the FAE (Bockman & Cooper, 1973; Owen & Jones, 1974) and the relative lack of goblet cells in the FAE compared with the villus eipthelium (Smith et al. 1980; Bockman & Cooper, 1973; Owen & Nemanic, 1978; Chu, Glock & Ross, 1979). The possibility exists that cells formed in the FAE might have their pattern of differentiation determined, in part, through close contact with underlying lymphoid tissue (Smith & Peacock, 1980). The ability of villus enterocytes to take up different amino acids, which is a late feature of functional differentiation, can now be studied at the cellular level by applying quantitative autoradiography to glutaraldehyde-fixed tissue (King, Sepulveda & Smith, 1981a, b). The purpose of the present work was to use this analytical 148 M. W. Smith and G. Syme technique with rat FAE to test whether lymphoid tissue might also affect the way amino acid transport function becomes expressed in enterocytes during the last stages of differentiation. MATERIALS AND METHODS Animals Rats used in the present work were of the Sheffield strain. Bred in Sheffield they were weaned at 21 days of age onto diets containing either 20 or 5 % protein, the carbohydrate content of the low protein diet being increased so that both diets remained isoenergetic (Syme, 1982). These animals had free access to tap water containing 3 fiM-Kl, to compensate for the lack of iodine in the diet, from the time of weaning. Experimental Animals were anaesthetized with an intraperitoneal injection of Sagatal (0-15 ml 100 g"1 body weight) and the small intestine was removed. Pieces of mid-jejunum, 10 cm long, were then cut open for mounting mucosal-face upwards in an uptake apparatus similar to that described previously (Sepulveda & Smith, 1978). This tissue was pre-incubated for 10 min in Na-free bicarbonate saline (Krebs & Henseleit, 1932), gassed with 9 5 % O , + s % C O t at 37 CC, before being used to measure vaJine uptake. Incubation with 1 mM-L-[3,4(n)-3H] valine (Amersham International Ltd, Amersham, U.K.; sp. act. 100 fid ml"1), for 45 s in Nacontaining bicarbonate saline, was stopped by washing with phosphate-buffered saline (Dulbecco 'A', pH7"3; Oxoid Ltd, Basingstoke, U.K.) containing 4 % glutaraldehyde and 2 % sucrose. Most of the fixed tissue consisted of intestinal villi but it was, on occasion, possible to detect the presence of Peyer's patches in localized areas. It was on this material that further analyses of valine uptake were carried out. Autoradiography Pieces of glutaraldehyde-fixed jejunum containing Peyer's patches, taken from the uptake apparatus after contact with tritiated valine for 45 s, were transferred to fresh glutaraldehydecontaining medium for 2 h before being washed twice in phosphate-buffered medium to remove fixative. The subsequent dehydration, embedding in glycolmethacrylate, cutting of 3-/*m sections, coating with photographic emulsion and development 12 days later, was as described previously for rabbit ileum (King et al. 1981 a, b). In further experiments designed to measure cell renewal time in villus mucosa, tritiated thymidine ([6-'H]thymidine; Amersham International Ltd, Amersham, U.K.) was injected intraperitoneally into rats (1 fid g~l body weight). These animals were killed 2, 10, 20, 30 or 40 h later and the mid-jejunum removed for fixation in phosphate-buffered saline containing 4 % glutaraldehyde and 2 % sucrose. The subsequent processing of these tissues for autoradiography was identical to that described above. It was routine practice to incubate all sections, following development and fixation, in silver intensifier solution (IN-5) for a period of 1 min to increase the size of silver grains. This improved the accuracy of subsequent analyses. Microdensitometry Scanning microdensitometry of unstained autoradiographs was carried out at a final magnification of x 1000 (x 10 eyepiece together with a x 100 oil-immersion objective) using a Vickers M85 microdensitometer (Vickers Instruments, York, U.K.). Scanning took place in discrete steps of 5 /*m moving from the centre of the FAE or the tips of adjacent villi towards their respective crypts. Errors in density reading, which could have arisen from scanning of goblet cells instead of enterocytes, were avoided by moving the scanning spot Enterocyte differentiation in rat jejunum 149 laterally into other areas of the epithelium. This operation was rarely needed in the FAE, however, where the number of goblet cells was found to be low compared with that found in the villus mucosa. Quantification of results Readings of optical density were converted into intracellular concentrations of valine by comparison with a known arginine standard, made up in gelatin and cut at the same section thickness as used for tissue. The water content of mucosal scrapings was found to be 80-3 ± 4-5 % (mean ± S.E. of 9 determinations). Gelatin standards were made up as a 20 % (w/v) solution in order to correspond to tissue water content. The specific activity of valine presented to the tissue was identical to that of arginine used as standard (100 fid ml"1). Optical densities obtained from tissue and gelatin sections were each corrected for losses arising from incomplete retention during glutaraldehyde fixation (66 and 54-5 % retention determined for valine and arginine, respectively, using methods of analysis described previously; King et al. 1981a, b). Final calculation of intracellular valine concentrations was done by dividing corrected tissue optical densities by that obtained for the standard. RESULTS Autoradiographic localization of valine uptake by rat follicle-associated epithelium Tritiated valine at a concentration of 1 mM was presented to the luminal surface of rat mid-jejunum for a period of 45 s, as described in the previous section. A typical autoradiograph of sectioned tissue taken in the region of a Peyer's patch is shown in Fig. 1 A. Radioactivity is seen to be concentrated in the dome region of the FAE and in the upper half of surrounding villi. These villi appear to take up significantly greater amounts of valine than does the FAE. A companion section of the same piece of tissue has been stained with haematoxylin and eosin to show the fine structure of this region of the intestine (Fig. 1 B). The almost spherical lymphoid follicle is covered by a layer of epithelium (the FAE). This epithelium is continuous with that covering adjacent villi. Previous work in the mouse shows this FAE to be derived from cells located in crypts situated around each follicle (Smith et al. 1980). Similar crypts are present around the edge of rat lymphoid follicles (Fig. 1 B). The rates used in these experiments had been maintained on a diet containing 5 % protein for a period of 4 weeks prior to experiment. The effect of diet on the pattern of amino acid uptake by the FAE is dealt with in a separate section. Quantitative analysis of valine uptake by rat FAE Microdensitometry was carried out on autoradiographs of rat FAE prepared in a similar way to that shown in Fig. 1 A, values of optical density being converted to intracellular valine concentrations as described in Materials and Methods. The results obtained are compared with those obtained from surrounding villi (Fig. 2). Radioactivity in the FAE remained close to background up to about 210/4m from the base of follicle-associated crypts. Valine uptake then began to increase steadily reaching maximum intracellular concentrations of about 5 mM at or shortly before the apex of the follicle had been reached. Uptake of valine by surrounding villi M. W. Smith and G. Sytne B * Fig. i • « * « Enterocyte differentiation in rat jejunum 01 0-2 0-3 Distance from crypt—villus junction (mm) 0-4 Fig. 2. A comparison of the ability of different areas of follicle-associated and villus epithelium to transport and concentrate valine during a 45-s incubation at 37 °C. Each value represents the mean of determinations carried out on 15 villi (—) or 4 follicles (--) respectively. The concentration of valine in medium presented to the jejunum is shown by the hatched area. remained negligible up to 160/fin from the base of villus-associated crypts. There was then a gradual increase in valine uptake, similar to that seen in the FAE, causing the intracellular concentration to double over the next 50 fim. of villus surface. This was followed by a further rapid increase in absorptive function leading to a sixfold rise in intracellular valine concentrations as the enterocytes migrated the last 90 /tm to the villus tip. A similar two-stage increase in amino acid absorption has been reported recently for rabbit ileum (King et al. 1981 b). All these results were obtained using pieces of mid-jejunum taken from rats maintained on a diet containing 5 % protein. Fig. 1. Serial sections of rat mid-jejunum showing the location of tritiated valine (A) and the general histological structure (B) of a rat lymphoid follicle and surrounding villi. Sections cut at 3 ftm were either processed for autoradiography (A) or stained with haematoxylin and eosin (B), as described in the text. Arrows show the position of follicle-associated crypts. Bar, 500 fim. M. W. Smith and G. Syme 152 Effect of diet on valine uptake by rat FAE Weanling rats kept for 4 weeks on a diet containing 20% protein produce villi that are approximately 50% longer than those found in litter-mates fed an isoenergetic diet containing 5% protein (Syme, 1982). These changes take place without affecting the size of associated lymphoid follicles (follicle diameter, about 500 /tm irrespective of diet). The following experiments were carried out to compare the ability of FAE taken from rats fed 20 % protein to take up valine with that seen using tissue taken from rats maintained on a low protein diet. 10 E 3 oi c x I 0-8 - ..•••' / «•) D / g / /I T a e o-4 a c / •act o J- I /^ ' 0-2 0 \d \ 10 i 20 i 30 40 50 Time(h) Fig. 3. Kinetics of cell replacement in rat mid-jejunum. Rats were killed after intra-peritoneal injection of tritiated thymidine. Processing of tissue and measurement of cell position on sectioned villi was as described in the text. Values give means ±s.E. of 10 separate determinations. Open arrow, time needed to completely replace mid-jejunum mucosa (51 h). Valine uptake by the FAE of tissues taken from rats fed 20% protein began, as with the animals fed on low protein diets, to reach levels that are significantly different from background some 200 fim. from the base of follicle-associated crypts. There was then a gradual increase in uptake as enterocytes continued to migrate towards the apex of the FAE. At this point, however, the total uptake of valine.was only about one fifth of that recorded using tissue taken from the animals on the low protein diet. This reduction in uptake was accompanied by an actual 12% increase in valine uptake measured at the tips of villi taken from rats maintained on the high protein diet. It is concluded from these experiments that changes in diet can alter the capacity Enterocyte differentiation in rat jejunum 153 of the FAE to absorb amino acids and that this can take place without noticeably affecting the general pattern of enterocyte differentiation. It should be pointed out, however, that the amount of valine entering the FAE of tissues taken from rats maintained on the high protein diet was so low that it made detailed analysis difficult. It was decided for this reason to carry out all subsequent experiments on rats maintained on the low protein diet. Thymidine-labelling experiments The process of differentiation whereby a structurally complete enterocyte begins to express absorptive properties has been described up till now in terms of cell distance from point of origin. Knowing the cell turnover time for villus epithelium, however (and assuming the same cell turnover time to apply to the rat FAE as it does in the mouse; Smith et al. 1980), allows one to relate amino-acid-absorptive function to the age of cell involved. In order to do this, tritiated thymidine was injected intraperitoneally into a group of rats, individual animals then being killed at known times afterwards. Pieces of mid-jejunum were processed for autoradiography and the highest position of a cell containing label was measured and expressed 15 20 25 30 35 40 45 50 Enterocyte age (h) Fig. 4. Relation between age of enterocyte and its ability to take up tritiated valine during a 45-8 incubation at 37 CC. Enterocytes were studied in follicle-associated epithelium (—) and in surrounding villi ( ). Each value gives the mean of determinations carried out on 4 follicles and 15 villi. 6 CELJ5 154 M. W. Smith and G. Syme as a fraction of the total villus height. The results obtained from these experiments are summarized in Fig. 3. Labelling was confined to the bottom of crypts 2 h after thymidine injection. These labelled cells were emerging from crypts onto villi some 8 h later. Migration of enterocytes along the whole length of villi was related linearly to the time after thymidine injection. Extrapolation of the last part of the graph gives an estimated time for cell renewal of 51 h. Assuming 51 h to represent the time needed for complete cell renewal in the FAE as well as the villus epithelium, it was calculated that enterocytes were 30 h old when they first began to absorb valine (Fig. 4). This applied whether the enterocyte came from a villus or the FAE. The rate at which valine entered enterocytes then began to increase at a constant rate for a period of 10 h, the intracellular concentration of valine in a 40-h-old enterocyte being about three times that presented originally to the rat jejunum. From then on the ability to concentrate valine diverged in the two tissues; enterocytes in the FAE increased their capacity to transport valine at the same rate as before, while villus enterocytes entered a final stage where uptake increased more rapidly. DISCUSSION There is already extensive literature supporting the idea that lymphocyte traffic across cell monolayers can affect their gross morphology. Some of these authors have studied the ability of lymphocytes to create a particular type of high endothelial cell in post-capillary venules (for references, see Goldschneider & McGregor, 1968), and endothelial cells showing this characteristic have already been identified in the interfollicular areas of Peyer's patches taken from both rats and mice (Schoefl, 1972; Anderson, Anderson & Wyllie, 1976; Abe & Ito, 1977). More recently it has also been suggested that lymphocyte traffic across the FAE of mouse Peyer's patches can affect the morphology of columnar enterocytes so that they begin to transport antigens (Smith & Peacock, 1980; Smith & Peacock, 1982). But do lymphocytes actually have to cross cell layers in order to produce these marked changes in cellular structure and function? The generally accepted finding that the FAE contains fewer goblet cells than villus epithelium (Bockman & Cooper, 1973; Owen & Nemanic, 1978; Chu et al. 1979), even though both types of tissue originate from closely associated crypts (Smith et al. 1980), suggests that something within follicles (possibly the lymphocyte) is capable of exerting much more specific effects on cell differentiation than had been previously recognized. This hypothesis is strengthened by the present finding that morphologically similar enterocytes in villus and follicular epithelium behave quite differently when asked to perform a common absorptive function. The general reduction in the capacity of follicular enterocytes to absorb valine, seen irrespective of diet, agrees with an earlier finding in rabbit Peyer's patch showing the transmural flux of different amino acids to be significantly less than that measured across a corresponding area of villus epithelium (Hajjar, Hawrani & Khuri, 1972). The present work allows one to interpret this kind of result in greater Enterocyte differentiation in rat jejunum 155 detail. We have found two phases of uptake in the villus epithelium but only one in the FAE. It is the absence of this second phase of carrier expression that results in the differences seen when measuring amino acid entry into these two tissues. Why FAE enterocytes fail to show this later stage of functional differentiation remains unclear. One suggestion could be that this aspect of enterocyte development needs to be initiated by factors only found within the core of the villus. Another suggestion could be that lymphocytes play a more active role in actually inhibiting a normal process of cell differentiation programmed to take place within migrating enterocytes. Though unproven, this latter suggestion would be consistent with the further finding that goblet cells are less readily formed from stem cells in the crypts of the FAE (Bockman & Cooper, 1973; Owen & Nemanic, 1978; Chu et al. 1979; Smith et al. 1980) and that close proximity of lymphocytes to fully differentiated enterocytes often leads to gross changes in microvillar structure (Smith & Peacock, 1980, 1982). It is hoped that the introduction of the present technique, allowing the functional characteristics of enterocytes to be monitored in the FAE throughout differentiation, might help to resolve some of these uncertainties in the future. We wish to thank Mr I. S. King for preparation of tissue sections for histological and autoradiographical analysis, and Mr A. L. Gallup for photography of intestinal tissue. REFERENCES ABE, K. & ITO, T. (1977). A qualitative and quantitative morphologic study of Peyer's patches of the mouse. Archt Histol., Japan 40, 407-420. ANDERSON, N. D., ANDERSON, A. O. & WYLLIE, R. G. (1976). Specialized structure and metabolic activities of high endothelial venules in rat lymphatic tissues. Immunology 31, 455-473BOCKMAN, D. E. & COOPER, M. D. (1973). Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix and Peyer's patches. An electron microscopic study. Am. J. Anat. 136, 455-478. CHU, R. M., GLOCK, R. D. & Ross, R. F. (1979). Gut-associated lymphoid tissues of young swine with emphasis on dome epithelium of aggregated lymph nodules (Peyer's patches) of the small intestine. Am. J. vet. Res. 40, 1720-1728. GOLDSCHNEIDER, I. & MCGREGOR, D. M. (1968). Migration of lymphocytes and thymocytes in the rat. I. The route of migration from blood to spleen and lymph nodes. J. exp. Med. 127, 155-168. HAJJAR, J.-J., HAWRANI, M. & KHURI, R. N. (1972). Alanine flux across rabbit Peyer's patches. Comp. Biochem. Pkysiol. 43 A, 723-732. KING, I. S., SEPULVEDA, F. V. & SMITH, M. W. (1981a). Autoradiographic analysis of amino acid uptake by rabbit ileal mucosa. J. Physiol., Lond. 316, 1-2P. KING, I. S.( SEPULVEDA, F. V. & SMITH, M. W. (19816). Cellular distribution of neutral and basic amino acid transport systems in rabbit ileal mucosa. J. Physiol., Lond. 319, 355-368. KINTER, W. B. & WILSON, T. H. (1965). Autoradiographic study of sugar and amino acid absorption by everted sacs of hamster intestine. J. Cell Biol. 25, 19-39. KREBS, H. A. & HENSELEIT, K. (1932). Untersuchungen iiber die Harnstoffbildung im TierkOrper. Hoppe-Seyler's Z. physiol. Chem. 210, 33-66. OWEN, R. L. & JONES, A. L. (1974). Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66, 189203. OWEN, R. L. & NEMANIC, P. (1978). Antigen processing structures of the mammalian intestinal tract: an SEM study of lymphoepithelial organs. In Scanning Electron Microscopy. Proc. Works on Adv. Biomed. Appl. of SEM, pp. 367-378. Chicago: IIT Research Institute. 6-2 156 M. W. Smith and G. Syme G. I. (1972). The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A re-examination J. exp. Med. 136, 568-588. SEPULVEDA, F. V. & SMITH, M. W. (1978). Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J. Pkysiol. (Lond.) 282, 73-90. SMITH, M. W., JARVIS, L. G. & KING, I. S. (1980). Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am.jf. Anat. 159, 157-166. SMITH, M. W. & PEACOCK, M. A. (1980).' M' cell distribution in follicle-associated epithelium of mouse Peyer's patch. Am.J. Anat. 159, 167-175. SMITH, M. W. & PEACOCK, M. A. (1982). Lymphocyte induced formation of antigen transporting ' M' cells from fully differentiated mouse enterocytes. In Second International Conference on Intestinal Adaptation, Lancaster: MTP Press Ltd. (In Press.) SVME, G. (1982). The effect of protein-deficient isoenergetic diets on the growth of rat jejunal muco9a. J. Nutr. (In Press.) SCHOEFL, (Received 22 October 1981)