* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmaceutical Task Group Marketing
Target audience wikipedia , lookup
Marketing communications wikipedia , lookup
Affiliate marketing wikipedia , lookup
Marketing research wikipedia , lookup
Marketing channel wikipedia , lookup
Digital marketing wikipedia , lookup
Integrated marketing communications wikipedia , lookup
Sports marketing wikipedia , lookup
Marketing strategy wikipedia , lookup
Ambush marketing wikipedia , lookup
Guerrilla marketing wikipedia , lookup
Youth marketing wikipedia , lookup
Viral marketing wikipedia , lookup
Advertising campaign wikipedia , lookup
Sensory branding wikipedia , lookup
Direct marketing wikipedia , lookup
Marketing plan wikipedia , lookup
Multi-level marketing wikipedia , lookup
Multicultural marketing wikipedia , lookup
Marketing mix modeling wikipedia , lookup
Global marketing wikipedia , lookup
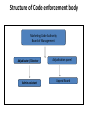
Medicines and Related Substances Amendment Bill 2008 Pharmaceutical Task Group: Industry Marketing Code Steering Committee Presented by: Maureen Kirkman & Jim Ringer (Chairperson) 5 August 2008 PTG – Pharmaceutical Industry Marketing Code Steering Committee 2007-2008 • • • • • IMSA NAPM PHARMISA PIASA SMASA Innovative Medicines South Africa National Association of Pharmaceutical Manufacturers Pharmaceuticals made in South Africa Pharmaceutical Industry Association of South Africa Self Medication Association of South Africa • Supported by: • • • • NAPW IHD PHD PSSA National Association of Pharmaceutical Wholesalers International Healthcare Distributors Pharmaceutical Healthcare Distributors Pharmaceutical Society of SA This initiative to eliminate perversities is supported by the entire medicines supply chain Medicines and Related Substances Amendment Bill 2008 Background • Many perversities in the marketplace related to the marketing of medicines and other health products • In 1998 attempt to remove these for medicines by amendments [Sec 18A] banning incentives and required the Minister of Health to make Regulations for the marketing of medicines and to provide for an enforceable Code of Practice [Sec 18C] • We welcome the application of these measures to the marketing of all health products to eliminate perversities Legal Gaps and Marketing Code • There is no provision for regulations to support S18A and this is creating loopholes and opportunities to get around the ban on incentives • No direct legal link between the Medicine Pricing Regulations and marketing incentives • Marketing Code for Medicines not published in an enforceable form by DoH • No enforcement • Perversities continue in the marketing of medicines and other health products – undermines the intent of the Medicines Pricing Regulations Amendment Bill 2008 • Industry supports the elimination of perversities in the system [ Section 18A and 18C ] • Publication of a code/s is essential – but • The Bill changes wording from ‘the MoH “shall” to “may” make Regulations’ relating to a Code of Practice for marketing of health products. This change should not be made in the Bill Reason: • Compliance must be mandatory for all companies and for all products to protect patients • Compliance must be enforced through the legislation and registration processes Recommendation • Empowering provision needed for Regulations [Sec 35] to enforce compliance with the legislation [18A] and Marketing Codes [18C] • Code to be based on self-regulation by industry and supported by ultimate enforcement via the SAHPRA • Compliance with the code to be a condition for registration of a medicine or a health product • Publish SA Marketing Code for Medicines without delay and follow with relevant Codes of Marketing Practice for other health products Conclusion Our request: • Provide for regulations to S18A and S18C • Finalise and publish SA Marketing Codes • Step wise implementation of Code – medicines first • Objective is to ensure ethical marketing practices and eliminate perversity in the marketing of health products to protect patients Contacts • • • • • • • • IMSA NAPM PHARMISA PIASA SMASA NAPW IHD PSSA Val Beaumont Raseela Inderlall Stavros Nicolau Maureen Kirkman Allison Vienings Trevor Phillips Murray Clark Ivan Kotze 011 012 011 011 012 083 011 012 880 323 239 805 803 378 458 301 4644 7529 6798 5100 4444 3260 2222 0833 Structure of Code enforcement body Marketing Code Authority Board of Management Adjudicator / Director Admin assistant Adjudication panel Appeal Board Composition of Board • Industry representatives – from companies and trade associations supporting the Codes • Dept of Health • Health professionals from HPCSA / SAPC • Consumer representative










![My_Body[1] - Junior2TopicWiki](http://s1.studyres.com/store/data/008060165_1-be31cd2568d5e2c9fee6ce67732b07b4-150x150.png)