* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Developing medicines for the future and why it is challenging
National Institute for Health and Care Excellence wikipedia , lookup
Compounding wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Drug interaction wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Neuropharmacology wikipedia , lookup
Patent medicine wikipedia , lookup
List of off-label promotion pharmaceutical settlements wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug design wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription costs wikipedia , lookup
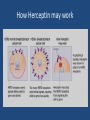
Developing medicines for the future and why it is challenging Angela Milne Fundamentals of medicines development • Quality • Safety • Efficacy Risk benefit assessment e.g. therapies for hay fever vs those for advanced cancer Controls on medicines development • Highly regulated process e.g. MHRA, EMEA, FDA • Stringent controls during drug development • Assessment process prior to approval to market • Value based judgements prior to availability to patients e.g. NICE, local formularies Process of drug developmentprediscovery • Select disease focus • Commercial viability e.g. cancer, respiratory, cardiovascular • Unmet medical need e.g. antibiotic resistance • Expertise of scientists • Gain understanding science of disease Process of drug developmentdrug discovery • Select target e.g. gene, protein and search for molecules/compounds to alter disease • Time frame 4 to 5 years • 5,000-10,000 compounds to achieve one approved medicine • Cost £436 million Process of drug developmentpreclinical • Early safety and efficacy tests in computational models, cells and animals • Time frame one year • 10 to 20 candidates to achieve one approved medicine • Cost £97 million Process of drug developmentPhase 1 clinical trials • Tested in human volunteers and patient volunteers (20-100). No therapeutic benefit • Time frame 1.5 years • 5-10 candidates to achieve one approved medicine • Cost £177 million Process of drug developmentPhase II • Evaluation of candidates drug efficacy in patients (100-500) • Time frame 1.5 years • 2 to 5 candidates to achieve one approved medicine • Cost £204 million Process of drug developmentPhase III clinical trials • Evaluation of candidate drug in patient population for marketed indication (10005000) • Generates efficacy, safety, and overall risk benefit data • Timeframe 2.5years • 1-2 candidates to achieve one approved medicine • Cost 1.84 million Licensing approval • Information & results from all studies compiled and submitted to regulatory agencies e.g. EMEA, FDA • Time frame 1.5 years • One approved medicine (if only!) • Cost £500,000 Drug development-total timeframe and cost • Average number of years to develop a successful medicine- 12.5 years • Average cost to research and develop a successful medicine- £1.15 billion Medicines available for patients • Value & cost effectiveness assessments e.g. NICE • Local health budget availability • Competitor products Drug Development –it doesn’t stop there! • Original approval often given for limited indication so repeat clinical trial process for new indications e.g. for cancer drug usually first approved for late stage cancer and then researched for earlier stage cancers • New formulations e.g. development of sustained release formulations • Development of improvements in manufacturing process Long term obligations • Conduct of any additional studies required by licensing authorities • Long term monitoring of safetypharmacovigilance • Meeting requirements of good manufacturing practice • Annual reporting requirements to regulatory authorities The future of medicines research • Conventional pathway of drug development has been successful for many years but the hugely increasing cost of drug development has not resulted in a similarly large increase in the number of new medicines • Additional techniques and approaches need to be explored to expand the range of methods to develop new medicines New techniques and approaches • • • • Flexible drug development process Increased collaboration Value and outcomes evaluation in trial design Personalised medicines Personalised medicines • Target treatments specifically to patient populations most likely to respond • Classify individuals into subpopulations that differ in their susceptibility to a particular disease or their response to a specific treatment • Concentrate preventative or therapeutic interventions on those most likely to benefit thus optimising patient benefit, sparing side effects and expense of treating those unlikely to benefit. • Involves development and use of companion diagnostics to achieve the best outcomes for the patient Targeted cancer therapy-hopes and challenges • Generally well tolerated and toxicity tends to be less severe than with conventional cytotoxic therapy • Targeted therapy is different from other types of therapy. Designed to target cells with specific receptors for treatment • Traditional therapies • • • -Radiation therapy uses high energy rays to kill cells and or shrink tumours -Chemotherapy is drugs that are used to destroy cells -Hormonal therapy helps fight tumours that thrive on hormones like estrogns by acting on hormone receptors on tumour cells or by decreasing the amount of estrogen available to bind these receptors Example of targeted therapy • Herceptin is used for treatment of certain Human epidermal growth factor receptor-2positive (HER2+) cancers e.g. breast cancer, gastric cancer. • HER2 testing is performed with the tumour sample removed during surgery or using a needle How Herceptin may work Targeted cancer therapy-hopes and challenges • Gefitininib is an epidermal growth factor receptor inhibitor. It was originally tested in NSCLC patients many of whom did not have EGRF positive NSCLC and did not show benefit over best supportive care. Seven years after initial study subgroup analyses revealed benefit in patients with activating mutations of EGRF and drug received licence for EGRFpositive patients. • Crizotinib, an anaplastic lymphoma kinase inhibitor only tested in the 5% of NSCLC was more effective than standard therapy in patients found to express the target enzyme. It has been licensed for this indication An integrated approach to the development of personalised medicines • Co-operation required between: • • • • • • • Pharmaceutical industry The diagnostics sector Research funders Regulators Healthcare providers and policy makers Health informatics programmes Health economics Discussion and questions • How long have you got!























![My_Body[1] - Junior2TopicWiki](http://s1.studyres.com/store/data/008060165_1-be31cd2568d5e2c9fee6ce67732b07b4-150x150.png)