* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download International Editorial Board
Survey
Document related concepts
Psychedelic therapy wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Potassium iodide wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Transcript
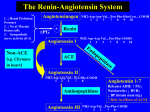
For Rational Drug Use and Disease Management SEPTEMBER 16, 1996 VOLUME 8, NO. 6 ISSN 1172-0360 CONTENTS New drugs and therapeutics 1 Losartan potassium: low incidence of cough confirmed 5 Which drug for stable angina? 9 Scabies still a widespread problem in many countries Drug reactions and interactions 11 Aplastic anaemia and ocular chloramphenicol: any link? Drug economics and quality of life 13 A place on the formulary for SSRIs in the treatment of depression Losartan potassium: low incidence of cough confirmed Losartan potassium is the first of a new class of antihypertensives, the angiotensin II antagonists. It has similar properties to those of ACE inhibitors, but unlike these drugs it does not appear to cause persistent cough. Thus, losartan potassium has a particular niche for use in patients with essential hypertension who cannot tolerate ACE inhibitors because of persistent cough. An expanded role in the treatment of hypertension is likely to require long-term outcomes data and pharmacoeconomic analysis. In addition, whether losartan potassium has similar benefits to ACE inhibitors in congestive heart failure and nephropathy awaits the results of ongoing s t u d i e s . Losartan potassium was first reviewed in Drugs & Therapy Perspectives in 1993 when it was in the clinical phase of development [1] The drug has now been marketed in a number of countries; thus, this review will evaluate the increasing body of clinical data on losartan potassium to consider whether its potential advantages have been realised. Losartan potassium was the first angiotensin II antagonist to reach the market; other drugs of this type include valsartan (launched in Germany), irbesartan (preregistration in Europe), and candesartan hexetil, eprosartan, telmisartan and tasosartan (all phase III). Losartan potassium is compared with enalapril in the Differential features table. A new class of antihypertensive Losartan potassium and its active metabolite E3174 inhibit the renin-angiotensin system more completely and more selectively than ACE inhibitors.[2] The drug acts specifically at the angiotensin II subtype 1 receptor (ATi), which appears to account for all the known cardiovascular effects of angiotensin II [2] It does not inhibit the AT2 receptor subtype, the function of which has not yet been defined. [2] INTERNATIONAL † Losartan potassium has been launched for treating essential hypertension in Canada, Denmark, France, Germany, Sweden, the UK and the US; it is not yet available in Australia. Drugs & Therapy Perspectives Editor: C. Rhoda Lee Associate Editor: Alison J. Forbes Editorial Secretary: Carol Milligan Group Editorial Director: Rennie C. Heel Publishing Director, Periodicals: Paul Chrisp Research Editors: Tracey Langsdale (Senior Editor);, Pauline Curel; Tracey Hale; Gill Higgins; June Katz; Gillian Keating; Tamara Schwarz; Theresa Szeto; Carlene Todd; Rachel Webber-Foster Editors, Clinical Pharmacology and Therapeutics: Sarah Edmonds (Senior Editor); Alan S, Beedle; Claire L. Berkahn; Stephen G. Coleman; Richard Crampton; Katharine J, Palmer; Rosie Stather Editors, Drug Evaluations: Donna McTavish (Senior Editor);, Julia A, Balfour; Paul Benfield; Rex N. Brogden; Harriet M. Bryson; Allan Coukell; Rick Davis; Christopher J. Dunn; Diana Faulds; Jane C. Gillis; Karen L. Goa; Malini Haria; Wendy Jeal; Heather D. Langtry; Andrew P. Lea; Anthony Markham; Stuart Noble; Caroline M, Perry; Greg L. Plosker; Caroline M. Spencer; Antona Wagstaff; Michelle I. Wilde; Lynda R. Wiseman Pesident and Publisher: Graeme S. Avery. International Editorial Board D.R. Abernethy, Washington, DC, USA; C,P.A Alderman, Adelaide, SA, Australia; C.D. Bayliff, London, Canada; EJ. Begg, Christchurch, New Zealand; T, Bergan, Oslo, Norway; G. Bianchi Porro, Milan, Italy; C, Brater, Indianapolis, IN, USA; A.M. Breckenridge, Liverpool, UK; W.W. Busse, Madison, WI, USA; S.G. Carruthers, London, Canada; G. Carstens, Hanover, Germany; J.A. J.H. Critchley, Hong Kong;R.O. Day, Sydney, NSW, Australia; C. Doreau, Paris, France; M.N.G. Dukes, Sorborg, Denmark; A. Ebihara, Tochigi-ken, Japan; L. Ereshefsky, San Antonio, TX, USA; S, Erill, Barcelona, Spain; S.Erush, Philadelphia, PA, USA; P. Fallet, Paris, France; J. Feely, Dublin, Ireland; R.G. Finch, Nottingham, UK; S. Frias, Madrid, Spain; W.H. Frishman, Bronx, NY, USA; S.T. Garner, Nottingham, UK; D.J. Greenblatt, Boston, MA, USA; R. Gugler, Karlsruhe, Germany; F.D. Hart, London, UK; D.A. Henry, Newcastle,, NSW, Australia; I. Hindmarch, Godalming, UK; T. Ishizaki, Tokyo, Japan; R. Janknegt, Sittard, The Netherlands; G.L.Kearns, Kansas City, MO, USA; J. Kenyon, Auckland, New Zealand; S,K. Lam, Hong Kong; M. Levy, Jerusalem, Israel; W, J. Louis, Heidelberg, Vic, Australia; H.I. Maibach, San Francisco, CA, USA; G. Mancia, Monza, Italy; A. McLean, Melbourne, Vic, Australia; P.A. Mitenko, Nanaimo, Canada; D. Nash, Philadelphia, PA, USA; M.O. Nielsen, Hurup, Denmark; P.L. Nielsen, Frederikshavn, Denmark; H.C. Neu, New York, NY, USA; L.P. Opie, Cape Town, South Africa; M. Packer, New York, NY, USA; R. Pauwels, Ghent, Belgium; P. Persson, Stockholm, Sweden; M.M. Reidenberg, New York, NY, USA; J. Schentag, Buffalo, NY, USA; D-R. Sohn, Chonan, Korea; De K. Sommers, Pretoria, South Africa; N.ATerragno, Buenos Aires, Argentina; M. Thomas, Vellorre, India; J-P. Tillement, Creteil, France; G. Tognoni, Milan, Italy, J.H. Toogood, London, Canada; C. Tugwell, London, UK; T. Uematsu, Gifu, Japan; C.J. van Boxtel, Amsterdam, The Netherlands; R.E. Vestal, Boise, ID, USA; H.B. Wong, Singapore See back cover for subscription details Vol. 8, No. 6; September 16, 1996 In contrast, ACE inhibitors act earlier in the reninangiotensin system by blocking the formation of angiotensin II from angiotensin I by inhibiting ACE. Because angiotensin II is also formed by other enzymes that are not blocked by ACE inhibitors (e.g. cardiac chymase), ACE inhibitors do not completely prevent the effects of angiotensin II.[2] Moreover, inhibition of ACE also results in accumulation of bradykinin, which has been implicated in causing ACE inhibitor-associated cough.[ 2 ] Whether angiotensin II antagonists are 'more than just ACE inhibitors without the cough' remains to be seen 24-hour duration of action Blood pressure reductions produced by once-daily losartan potassium 50 to 100 mg/day persist throughout 24 hours as assessed by ambulatory blood pressure monitoring [2] The drug does not affect heart rate. Single doses of losartan potassium and captopril (both 50mg) yielded similar quantitative effects on blood pressure, although the onset was a few hours later with losartan potassium. [2] The effect of losartan potassium on other haemodynamic or cardiac indices in patients with hypertension has not been well documented. [2] Other effects In patients with hypertension, losartan potassium 100 mg/day increases plasma renin activity and plasma angiotensin II levels and appears to decrease plasma levels of aldosterone, at least in the short term.[5] Enalapril 20 mg/day increases plasma renin activity, but decreases plasma angiotensin II levels [5] Forearm vasodilator responses to bradykinin are not altered by losartan potassium, whereas they are potentiated by enalapril[2] In animal studies, angiotensin II receptor antagonists have been shown to: • prevent cerebrovascular infarcts • increase survival in chronic heart failure • slow progression of diabetes-related renal disease • regress left ventricular hypertrophy. [2] Preliminary data suggest losartan administration is associated with regression of left ventricular hypertrophy in patients,[2] but the other effects have yet to be demonstrated in humans, Pharmacokinetocs The bioavailability of losartan potassium is about 33%, indicating a considerable first-pass effect, and is not altered significantly by the presence of food. [2] In 1172-0360/96/00018-002/$01.00 Adis Internationa! Limited. All rights reserved most patients, about 14% of an oral dose of losartan potassium is metabolised via hepatic carboxylation to the active metabolite E3 174, which is more active than the parent drug.[2] However, in a very small proportion of patients (<1%), enzymes responsible for metabolism to E3174 are deficient (<1% of a dose is converted); [2] these patients may not respond adequately to the drug. Pharmacokinetic studies using cimetidine and ketoconazole suggest that clinically significant interactions with losartan potassium and CYP34A inhibitors are unlikely to occur. [2] However, drugs that induce cytochrome P450 systems may hasten the metabolism of losartan potassium, the clinical relevance of which has not been determined. No significant drug interactions appear to occur between losartan potassium and digoxin or warfarin. [2] Clinical efficacy Losartan potassium has demonstrated efficacy in the treatment of hypertension in randomised, double-blind studies, usually of 8 to 12 weeks' duration, involving about 3700 patients [2] As monotherapy . . . Large dose-finding trials have shown the efficacy of losartan potassium 50mg once-daily to be superior to that of placebo and indistinguishable from that of the l00 mg dose, [2] The drug has reduced diastolic blood pressure (DBP) by about 8 to 13mm Hg in patients with mild to moderate hypertension. [2] Antihypertensive efficacy, as determined by trough blood pressure values and percentage responders, has not differed significantly between once-daily administration of losartan potassium 50 to l00mg and enalapril 20mg, [7] atenolol 50 to l00mg[8] or felodipine extended release (ER) 5 to l0mg[2] in comparative trials. Blood pressure lowering was evident within 1 to 2 weeks of initiating losartan potassium therapy and was maximal by 3 to 6 weeks. [2] In the comparison with enalapril 20 mg/day, losartan potassium 50 mg/day appeared to be less effective according to an 'all patients treated' analysis (n = 399), but similarly effective by a 'per protocol' analysis that included only those patients who had a trough blood pressure measurement between 22 and 26 hours after the previous dose (n = 275). [7] . . . and combined with hydrochlorothiazide The combination of losartan potassium plus hydrochlorothiazide produces larger reductions in blood pressure than are seen with either drug alone. Compared with losartan potassium or hydrochlorothiazide monotherapy, 1172-0360/96/00018-003/SO 1.00 ®Adis International Limited. All rights reserved losartan potassium plus hydrochlorothiazide reduced DBP by an additional 4 to 6mm Hg. [2] During one 12-week noncomparative trial, severe hypertension was successfully managed in approximately 30% of patients treated with losartan potassium 50 or 100 mg/day plus hydrochlorothiazide 12.5 or 25 mg/day; another 22% of patients were maintained on losartan potassium monotherapy and the remainder required the addition of atenolol and/or a calcium antagonist.[2] Overall decreases in DBP Vol. 8, No. 6; September 16, 1996 a frequency of 3.1% for losartan potassium, 2.6% for placebo, and 8.8% for ACE inhibitors. [11] In trials conducted specifically in patients with a history of ACE inhibitor-related cough, the incidence of cough was similar for losartan potassium (17 to 29%), placebo (35%) and hydrochlorothiazide recipients (25 to 34%), but was much less than in lisinopril recipients (62 to72%).[16,17] Dosage and administration for regimens containing losartan potassium were in the magnitude of 19mm Hg in this population. Good tolerability Results from double-blind trials involving >2800 patients with hypertension who received losartan potassium indicate that the drug is well tolerated.[11] Although headache (14.1% of patients), upper respiratory tract infections (6.5%), dizziness (4.1%), asthenia/ fatigue (3.8%) and cough (3.1%) have been reported during administration of losartan potassium monotherapy, dizziness was the only adverse event documented more often with losartan potassium than with placebo treatment.[11] Orthostatic effects and first-dose hypotension can occur with losartan potassium therapy (combined incidence of 0.5% with 25 or 50 mg/day and 2.2% with 100 mg/day).[11] Liver enzyme levels have risen to a minor extent in 1.9% of losartan potassium-treated patients.[11] Hyperkalaemia (serum potassium 5.5 mmol/L) was documented in 1.5% of patients, none of whom required discontinuation of losartan potassium therapy. Losartan potassium appears to have a uricosuric effect.[2,7] To date, there have been 2 reports of angioedema.[12,13] 1 report of severe migraine[14] and 1 report of reversible loss of taste[15] developing during losartan potassium therapy. Low incidence of cough confirmed Clinical experience thus far has validated the expectation that losartan potassium would be associated with minimal cough because it lacks apparent effects on bradykinin.[2] Spontaneous reports of cough occurred at Vol. 8, No. 6; September 16, 1996 The recommended initial and maintenance dosage of losartan potassium as monotherapy in patients with essential hypertension is 50mg once daily. Some patients may benefit from 100 mg/day. Losartan potassium may be given with or without food.[2] In patients at high risk of hypotension or volume depletion and in those with hepatic dysfunction, the i n i t i a l dose should be 25mg.[2] No dosage adjustment is needed for the elderly or patients with renal impairment. Losartan potassium is not recommended for use in pregnant women because of the risk of fetal morbidity/mortality.[2] Treatment with the combination product containing losartan potassium and hydrochlorothiazide is initiated at a dosage of 50/12.5 mg/day which can be doubled if the result is unsatisfactory. This therapy is not recommended for use as initial treatment when monotherapy would suffice, for patients with hepatic impairment or for those with creatinine clearance 30 ml/min [2] Prescribing and formulary considerations Guidelines for the pharmacological management of hypertension in the US[18] and the UK[19] recommend initiation of therapy with diuretics or -blockers - these agents have been proven to reduce cardiovascular morbidity and mortality. [2] ACE inhibitors, 1-blockers and calcium antagonists are positioned as 'alternative firstline' strategies in patients in whom diuretics and -blockers are ineffective, contraindicated or poorly tolerated [2] The final position of losartan potassium among the various classes of drugs available for the management of hypertension awaits clarification from long-term outcomes data. Several important features of the profile of losartan potassium that require examination are: • its effects, if any, on mortality in hypertension • its potential for benefits on symptoms and mortality in congestive heart failure • its potential for renal benefits in diabetic nephropathy • whether long-term unopposed angiotensin II block ade, which exposes other angiotensin receptors to increased circulatory angiotensin II, will produce unwanted consequences 11 72-0360/96/00018-004/ O1.00 Adis International Limited. All rights reserved • whether its lack of effect on bradykinin will mean that it lacks some of the positive as well as the negative effects of ACE inhibitors • its cost effectiveness and effects on quality of life. [2] A number of studies are underway including the Eval uation of Losartan in the Elderly (ELITE) trial and the Losartan Intervention For Endpoint in hypertension (LIFE) trial. [2] Pending long-term efficacy, tolerability and mortality data, the drug is likely to be used primarily in patients who are not well managed with, or are intolerant of, their current therapy. [2] References 1. Losartan potassium: a more direct approach to lowering BP. Drug Ther Perspect 1993 Aug 30; 2 (4): 7-8 2. Goa KL, Wagstaff AJ. Losartan potassium: a review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. Drugs 1996 May; 51 (5): 820-45 3. British National Formulary No. 31. London: The Pharmaceutical Press, 1996 Mar 4. 1995 Physicians GenRx. St Louis, Missouri: Mosby-Year Book, Inc., 1995 5. Goldberg MR, Bradstreet TE, Mc Williams EJ, et al. Biochemical effects of losartan, a nonpeptide angiotensin II receptor antagonist, on the renin-angiotensin-aldosterone system in hypertensive patients. Hypertension 1995 Jan; 25: 37-46 6. Timmermans PBMWM, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993; 45: 205 7. Tikkanen I, Omvik P, Jensen H/E, et al. Comparison of the angiotensin II antagonist losartan with the angiolensin converting enzyme inhibitor enalapril in patients with essential hypertension. J Hypertens 1995 Nov. 13: 1343-51 8. Dahlof B, Keller SE, Makris L, et al. Efficacy and tolerability of losartan potassium and atenolol in patients with mild to moderate essential hypertension. Am J Hypertens 1995 Jun; 8: 578-83 9. Chan JCN, Critchley JAJH, Lappe JT, et al. Randomised, doubleblind, parallel study of the anti-hypertensive efficacy and safety of losartan potassium compared with felodipine ER in elderly patients with mild to moderate hypertension. J Hum Hypertens 1995; 9:765-71 10. Dunlay MC, Fitzpatrick V, Chrysant S, et al. Losartan potassium as initial therapy in patients with severe hypertension. J Hum Hypertens 1995; 9: 861-7 11. Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensinconverting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol 1995 Apr 15; 75: 793-5 12. Merck & Co., Inc. Losartan potassium prescribing information. West Point, PA 19486, USA, April, 1995. 13. Acker CG, Greenberg A. Angioedema induced by the angiotensin II blocker losartan [letter]. N Engl J Med 1995 Dec 7; 333: 1572 14. Ahmad S. Losartan and severe migraine [letter]. JAMA 1995 Oct 25; 274 (16): 1266-7 15. Schliengcr RG, Saxcr M, Haefeli WE. Reversible ageusia associated with losartan [letter]. Lancet 1996 Feb 17; 347: 471-2 16. Lacourciére Y, Brunner H, Irwin R, et al. Effects of modulators of the renin-angiolensin-aklosterone system on cough. J Hyperlens 1994 Dec; 12: 1387-93 17. Merck Cozaar cough advantage over ACE inhibitors labeling claim. FDC Rep Pink Sheet 1996 Jan 8; T&G 2 18. Sever P, Beevers G, Bulpitt C, et al. Management guidelines in essential hypertension: report of the second working party of the British Hypertension Society. BMJ 1993 April 10; 306: 983-7 1172-0360/96/00018-005/$01.00® Adis International Limited. All rights reserved 19. Joint National Committee on Detection Evaluation and Treatment of High Blood Pressure. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med 1993; 153: 154-83 Which drug for stable angina? Drug treatment is the therapy of choice for patients with chronic stable angina. [1] The 3 most commonly used drug classes are nitrates, -blockers and calcium antagonists; the choice between these will often depend on what concurrent disease the patient has. Angina Angina is a symptomatic manifestation of ischaemic heart disease, which is usually caused by severe atherosclerotic eccentric lesions of 1 or more of the coronary arteries. Symptoms occur when myocardial oxygen demand is greater than supply, and this is normally provoked by exercise or stress. Angina is classified as stable when the frequency of attacks remains reasonably constant for a period of a least 1 month.[1] Nitrates, calcium antagonists and -blockers appear equally effective in preventing angina attacks; choice is likely to depend on concomitant disorders Angina is a disease predominately of the elderly; 10 to 25% of the elderly population aged >70 years have been reported as having angina compared with only 2% of the population aged >30 years.[2] Because the population is mostly elderly, the occurrence of patients with coexisting medical conditions is high. [2] Modification of risk factors Risk factors for angina include smoking, obesity, diabetes mellitus, hypertension and hyperlipidaemia.[3] Thus, counselling patients to help them reduce body weight (particularly by reducing dietary fat intake), to stop smoking, and to take appropriate exercise is important. [1,4] Moreover, appropriate treatment of diabetes mellitus, hypertension, hyperlipidaemia and heart failure all increase the survival rate in patients with angina. [2] Improvement in angina can also be achieved by treating exacerbating conditions such as anaemia and hyperthyroidism.[2] Treatment for acute attacks Acute attacks of angina are nearly always treated with sublingual nitroglycerin (glyceryl trinitrate), which pro- Vol. 8, No. 6; September 16, 1996