* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Topic #19: Static Electricity and The Electric Field
History of electromagnetic theory wikipedia , lookup
Electron mobility wikipedia , lookup
Elementary particle wikipedia , lookup
Speed of gravity wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Electromagnetism wikipedia , lookup
Field (physics) wikipedia , lookup
Fundamental interaction wikipedia , lookup
Magnetic monopole wikipedia , lookup
Maxwell's equations wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Lorentz force wikipedia , lookup
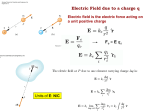
Topic #19: Electrostatics and Its Applications Part I: Static Electricity 1. The Electrical Atom 2. Transferring Electrons 3. Conductors, Insulators, and Semiconductors 4. Forces on Charged Bodies 5. Charging by Induction 6. Coulomb's Law 7. The Unit of Charge: The Coulomb 8. Forces on Neutral Bodies Part II: The Electric Field 1. Electric Fields 2. Electric Field Intensity 3. Work and the Electric Potential 4. The Electric Field Between Two Parallel Plates 5. Millikan's Oil Drop Experiment 6. Sharing of Charge 7. Electric Fields Near Conductors 8. Storing Charges: The Capacitor Notes should include: Part I: Static Electricity The Electrical Atom: J. J. Thompson discovered the electron. This was the first subatomic particle to be found. About ten years after the beginning of the 20th century a scientist from New Zealand discovered the positive nature of the nucleus due to the presence of protons. This person's name was Ernest Rutherford. By the early 1930's another gentleman by the name of James Chadwick, a student of Rutherford confirmed the existence of the neutron. So historically the nature of the atom was well on the road to being understood well before World War II (1941 to 1945). The atom has a dense center where most of the mass is found primarily in the form of protons (positive particles) and neutrons (neutral particles) with the very low mass electrons (negative particles) traveling around the outside of the nucleus producing the electron cloud. The proton and the neutron have about the same mass. The neutron is only slightly more massive. The electron has a mass only a little larger than 1 / 2000 of the mass of the proton or neutron. Transferring Electrons: Since electrons are on the surface of the atoms that make up materials around us, it is the addition of or the removal of electrons from materials that gives objects charge. Normally most objects are neutral. They have equal numbers of electrons and protons where each electron cancels the charge of a proton. The removal of electrons gives an object a positive charge, because of the imbalance of charge caused by electrons that are no longer present to balance some of the protons. The addition of electrons gives an object a negative charge, because this produces an excess of electrons that have no protons to cancel their charge. Electric charge is often produced through the use of the force of friction. On the other hand, electricity, www.physicsphenomena.com / Electrostatics and Its Applications 1 which is the flow of charge, begins as the accumulation of charge, then becomes electric current when it is allowed to flow through a circuit. Two classic examples of charge acquisition are described here. The first example involves taking a glass rod and rubbing it with a piece of silk cloth. The electrons in the atoms on the surface of the glass rod are not held as tightly as the electrons in the atoms on the surface of the silk. As a result, the glass rod acquires a positive charge while the silk cloth acquires a negative charge. The second example involves taking a rubber or plastic rod, even an ordinary comb would work, and rubbing it with a piece of fur. The electrons in the atoms at the surface of the rubber (or plastic) rod are held more tightly than the electrons in the atoms on the surface of the fur (or plastic). As a result, the rubber (or plastic) rod acquires a negative charge while the fur acquires a positive charge. Conductors, Insulators, and Semiconductors: When studying electric charge and electricity there are three terms you need to know. The first is conductor. A conductor is a material, such as a metal, that will allow electrons to move easily through the material. The second term is insulator. An insulator is a material through which electrons do not move. In fact rubber is an insulator. If you put a charge on a piece of rubber it tends to stay put where you put it. The charge is not likely to migrate very much through the rubber. The third term is semiconductor. A semiconductor is a material, such as germanium and silicon, that has a conducting capacity somewhere between a conductor and an insulator. In a semiconductor not as many electrons are free to move as in a conductor. Forces on Charged Bodies: Some important facts about charge include the fact that there are two kinds of charge, positive and negative. Also, charges exert forces on other charges over distance. And finally, like charges repel and unlike charges attract. To detect charges a device called an electroscope was invented. An electroscope is a device used to detect the presence of charge. It consists of a rod with a knob at the top and two small very thin metal leaves at the bottom. This rod is suspended in a frame or cabinet that keeps it insulated from everything around it including the cabinet itself. If the electroscope acquires a charge, the leaves spread out repelling each other. If the electroscope shows leaf repulsion when it is brought near to or in contact with another object, you know that object has a charge on it. The electroscope, however, does not distinguish as to whether the object has a positive charge or a negative one. It only shows whether there is a charge present. There are two ways to make the leaves separate due to repulsion. One means is uses charging by conduction. In charging by conduction, you actually touch the object to the knob at the top of the electroscope. If the object is positively charged, electrons from the electroscope will flow onto the object leaving the electroscope positively charged. The two positively charged leaves will separate. If the object is negatively charged, electrons will flow onto the electroscope giving the electroscope a negative charge. The two negatively charged leaves will separate. The second way to get an electroscope to reveal if a charge is present on an object is to use charging by induction. Charging by Induction: Charging by induction requires that the object be brought near the knob of the electroscope, but not actually touch it. If a positively charged object is brought close to the knob, it attracts electrons and the electrons will flow up into the knob. Since no contact is made, they do not actually leave the electroscope. However, by inducing this negative charge on the knob, the leaves now have an induced positive charge, because electrons have left the leaves. This www.physicsphenomena.com / Electrostatics and Its Applications 2 induced positive charge on the leaves causes the leaves to repel. Likewise, if an object having a negative charge were brought quite close to the knob, the negative charge would push electrons on the knob to move downwards away from the knob causing an induced positive charge to exist on the knob. Of course the electrons have moved onto the leaves giving them an induced negative charge, and naturally the leaves will separate. The most noticeable difference between an actual charge and an induced charge is observed when the object is taken away from the electroscope. An actual charge will remain on the electroscope keeping the leaves separate until the charge is allowed to leak off. An induced charge immediately disappears when the charged object is pulled away, because no exchange of electrons ever occurred. Coulomb's Law: Near the end of the 18th century, a French physicist by the name of Charles Coulomb used a torsion balance to determine the force between two charged spheres. This device is similar to the device used by Cavendish to verify the existence of gravitational force except that Coulomb was dealing with electrical force and not with gravity. He placed a sphere on each end of a rod made from an insulator. The spheres themselves were made of a conducting material. The rod was suspended from a thin wire. To find out how much the magnitude of the charges and distance between them affected the force between two charged objects he used a third sphere. This sphere could be charged the same as the ones on the rods or differently and could be moved around with respect to the distances between spheres. He observed the amount of twist or force experienced in the wire as he changed the variables of charge and distance. Finally, He came to two important conclusions, which are both related to the force between charged objects. His conclusions say that the size of the force that one sphere with a charge exerts on a second sphere with a charge (not necessarily the same charge) is proportional to the individual charges and is inversely proportional to the square of the distance between them. The equation which represents Coulomb's Law can be written as F = K q1 q2 / d2. The letter q represents the charge and d the distance between the two charged objects. The letter K is a numerical constant. Its value depends upon the units used in the calculation. The Unit of Charge, The Coulomb: The unit of charge is called the coulomb. If we reference this to the solitary charge on an electron called the elementary unit charge, the value of the charge on an electron expressed in coulombs is 1.60 x 10-19 C. An interesting question comes to many people's minds upon seeing this value. "How many electrons would produce one coulomb of charge. You can find this value by taking the reciprocal of the elementary unit charge expressed in coulombs. The answer you should get is 6.25 x 1018 electrons. Forces on Neutral Bodies: A charged body will repel another charged body with the same kind of charge. (two positives or two negatives) A charged body will attract another charged body with the opposite kind of charge. (one positive and one negative) A charged object will attract a neutral object, because of charge induction. Even insulators, though not to the extent of conductors, will experience some charge separation when a charged object is brought near them. As to whether a charged object can cause another object to actually move would be a function of the electrical force between the two objects and any resistant forces such as friction or weight. Part II: The Electric Field Electric Fields: Suppose you place a point charge (this means the object having the charge has no significant volume itself, but is like a single point in space) at some location. This charge will affect other point charges brought near it. The force between this original point charge and www.physicsphenomena.com / Electrostatics and Its Applications 3 another charge brought near it will be, as described by Coulomb's law, a function of the magnitude of the two charges and the distance between them. You can imagine a three dimensional space (volume) around this point charge where it will have an influence upon other charges. This region contains theoretically a huge number of positions where other charges could be placed and be influenced by this original point charge. This region, or sphere of influence, where the point source can influence other charges can be described as an electric field. This means that if you place a charge of known quantity at a certain distance from the point charge at the center of this field you can easily predict the forces magnitude and direction that is produced by the effect of the two charges being near one another. Often this electric field is mapped out and made into a visible model by using vectors drawn around the point charge to represent electrical field lines. Quantitatively, the magnitude of these vectors can be determined by imagining a test charge, such as a positive test charge of one unit of magnitude, at each of these positions. Instead of drawing vectors for each point as you move outwards in all possible directions, electric field lines are used. Electric field lines are lines drawn to visualize the direction of force around a point charge or between two or more charges placed near one another. Arrows are drawn on these lines to indicate the direction of force. The arrows point outwards away from positive charges and inwards towards negative charges. Remember that electric fields are real, but electric field lines are not. They are just a means of modeling an electric field. Electric Field Intensity: The ratio of the force to the charge is called the electric field intensity. This ratio's value is based on the test charge placed in the field and not the original charge in whose field the test charge is placed. The equation is written as E = F / q (of test charge). Work and Electrical Potential: If a positive test charge is placed in the electric field of a negatively charged point charge, the force will be negative and attractive. (Its negative, because for an attractive force to exist between two charges, one charge would be negative while the other would be positive.) If you attempt to move this test charge further out from the point charge, the attractive force will resist the motion and work will have to be done to move it. (remember: W = F d) The work done is proportional to the charge that is being moved. The difference in electrical potential between the two points (the first being the original position of the test charge and the second being the new location of the test charge after it was moved) is defined as the work done per unit charge. The equation can be written as Vpos2 - Vpos1 = W / q. The work done to move the charge out further from the point charge is positive, so the potential at the second position is larger than the potential at the first position. Vpos2 > Vpos1. The unit of electrical potential is the joule per coulomb. One joule per coulomb is called a volt. 1 V = 1 J/C. If the test charge were to be moved back to its initial position, the work done to move it would be negative. Also, in that situation the change in electrical potential will be negative. It should be noted that though the sign of the work changed, because the direction changed, the magnitudes of the work done first to move the charge outwards from position 1 to position 2 and the work done second to move the charge back to position 1 are the same. From this observation, it appears that the potential a test charge has when it is a specified position only depends upon its position. It should be emphasized that what is important here is that the individual potentials of a single point are not that important. What is important is changes or differences in potential. Only these differences are related to work being done. In studying electricity and electrical charge these potential differences are measured using a voltmeter. Since only the difference is significant, you www.physicsphenomena.com / Electrostatics and Its Applications 4 do not have to worry about an absolute zero potential, such as the location of the point charge. You may arbitrarily define one position, such as position 1 in our example, as having a zero value. Electrical potential between two unlike charges increases as the distance between them increases. In the case of like charges it is just the opposite. Work is done to push two like charges towards one another. The potential increases as the two charges are brought closer together. Since the change in potential varies directly with the work done and the work done varies directly with the electrical field intensity (because they both vary directly with force), the potential difference (change in potential) varies directly with the electrical field intensity. The Electrical Field Between Two Parallel Plates: If two flat plates of a conducting material are placed parallel to each other, you can produce an electric field that has uniform intensity. One plate is positively charged while the other is negatively charged. In a uniform electrical field the work done to move a charge a certain distance is defined by the equation W = f d. Next this equation for work can be substituted into the equation for electrical potential, such that the equation V = W / q becomes V = F d / q. And finally, recalling that the electric field intensity is E = F / q , the equation for electrical potential can be rewritten as V = E d. Millikan's Oil Drop Experiment: An American Physicist by the name of Robert A. Millikan, back in 1909, used the uniform electric field produced between two plates to measure the charge on a single electron. He sprayed tiny drops of oil into the air space between two charged electric plates. The oil drops often become electrically charged due to friction as they are shot out of the atomizer, the device he used to place the drops between the plates. Such a device is often used to dispense liquids like perfume. When the top plate was charged, those drops, which were negatively charged, would rise towards the positively charged plate. Millikan adjusted the potential difference between the plates for the purpose of suspending a drop between the plates. At this point the upward force of the electric field and the downward force of gravity acting on the oil drop were equal. From the equation for electric field intensity E = F / q, we get F = E q, and from Newton's second law and the fact that weight itself is a force we get F = ma = m g. Since the two forces are equal in this oil drop experiment, the two equations can be set equal to each other and a new equation can be written E q = m g. With this equation you can solve for the charge q on the electron, if you know the values for E, m, and g. His experimentation eventually yielded a value of -1.69 x 10-19 C for the charge of the electron. Sharing of a Charge: If a charged sphere is brought in contact with a second identical noncharged sphere the charge will be distributed across both spheres with each sphere having only half of the charge that the first sphere had originally. Initially the sphere with the charge has relatively high electrical potential, while the uncharged sphere has a potential of zero. After the charge is shared between them, the potential on each would be the same, but significantly less because each sphere now has a lesser charge. Now suppose you have two spheres, one of which is larger than the other, but they both have the same charge. The smaller sphere will have a greater electrical potential, because the like charges are closer together on this smaller sphere than on the larger one, that is, the closer together like charges are the higher their electrical potential. Though the two spheres have the same amount of charge, some of the charge on the smaller sphere would flow onto the larger sphere, if they were brought into contact, because of the difference in www.physicsphenomena.com / Electrostatics and Its Applications 5 electrical potential. The outcome is that the larger sphere ends up with a greater charge than the smaller sphere when the two have equal electrical potentials. The earth is such a large sphere that it takes on charge from objects touching it without any significant change in its electrical potential. In fact the process of touching a charged object to the ground to rid of charge is called grounding the object. For this reason we run wires from appliances to a metal stake driven as much as eight feet into the ground so any excess charge build up on the appliance bleeds off into the ground and doesn't end up traveling through us. As an alternative we might connect the appliance to our water pipe system because it also runs under the ground and if the ground is installed properly will allow the appliance to be grounded to the earth as well. We also want to be sure our computers and other sensitive equipment is grounded, because static electricity, a term used for a charge on an object, is not good for delicate electronic devices. It would be very easy for the static electricity to become an electron flow (electricity) and short out the computer, perhaps destroying its circuits in the process. Ground wires are also connected to fuel trucks whenever fuel is being added to or removed from tanks such as the ones below ground at gasoline stations, because a build up of static electricity could produce a spark which could ignite the fuel and blow up the truck, and the station. Electric Fields Near Conductors: As described above, the like electric charges on an object tend to spread out as far apart as is possible, thus making the electrical potential as low as is possible for a specific defined set of circumstances. This puts the charges on the surface of an object and not on the inside where they would be more crowded together. Electric fields around objects depend somewhat on their structure and shape. A spherical object that tapers to a point on one side will have more charge accumulate near the point. The field is strongest near the sharp points on the body. The electric field by these pointed regions of an object carrying a charge can become strong enough to ionize air molecules. When the ions recombine a spark is formed. For safety reasons highly charged conductors are made with rounded edges to reduce this problem. An exception is a device such as a lightning rod, where you want a conducting path to form between the rod and a cloud. The rod itself is connected to the ground via heavy wire. You want to attract the lightning bolt (a very big spark) to the ground where it will dissipate harmlessly without starting a fire or injuring someone. Storing Charges, Capacitance: Capacitors are devices used in electrical circuits to store charge. Back in his "kite flying" days Benjamin Franklin used Leyden Jars (a relatively primitive capacitor) to store the electric charge from lightning bolts. A common capacitor used today involves conducting plates separated by an insulator. A very simple one could be made of two metal plates separated by a layer of air. A capacitor has a constant q / V ratio. This ratio is called the capacitance of the capacitor. Capacitance is the ratio of the charge on either plate of a capacitor to the potential difference between the plates. The equation for capacitance is written as C = q / V. The unit of measure for capacitance is the farad, whose symbol is F. This letter is used to honor a scientist by the name of Michael Faraday who is known for his work with electricity. Vocabulary: Part I electrostatics, neutral, positive charge, negative charge, insulator, conductor, semiconductor, electroscope, charging by conduction, charging by induction, Coulomb’s Law, coulomb, elementary charge; Part II electric field, electric field lines, electric potential difference, volt, equipotential, grounding, capacitance, capacitor www.physicsphenomena.com / Electrostatics and Its Applications 6 Skills to be learned: 1. Describe the process of charging common objects 2. Describe how the electroscope is used to detect electric charge 3. Solving Coulomb's Law problems with two charges 4. Solving Electric Field Intensity problems 5. Describe how we picture an electric field 6. Describe the relationship between energy and electrical potential 7. Solving Electric field between two parallel plate problems 8. Describe the Millikan's Oil Drop Experiment 9. Solving Capacitance problems. Assignments: Textbook: Read / Study / Learn about electric charge and it applications WB Exercise(s): PS#20-1, PS#21-1, PS#21-2 Activities: TBA Resources: This Handout and the Overhead and Board Notes discussed in class Textbook: Chapters 20 and 21 WB Lessons and Problem Sets www.physicsphenomena.com - “Electrostatics and Its Applications” www.physicsphenomena.com / Electrostatics and Its Applications 7