* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Muscle contraction
Cyclic nucleotide–gated ion channel wikipedia , lookup
Chemical synapse wikipedia , lookup
Action potential wikipedia , lookup
Purinergic signalling wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Membrane potential wikipedia , lookup
Signal transduction wikipedia , lookup
P-type ATPase wikipedia , lookup
Cytokinesis wikipedia , lookup
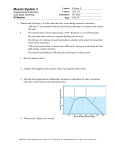
Metabolism of actively contracting muscles Fuel Utilization in Prolonged Exercise Fuel O2 utilization Fuel Concentrations in Blood (mM) Blood-delivered Fuels Exercise Time (min) Muscle Glyc ogen Glucose Fatty Acid 0 Glucose Lactate Fatty Acid Glycerol 4.5 1.1 0.66 0.04 4.6 1.3 0.78 0.19 40 36% 27% 37% 90 22% 41% 37% 180 14% 36% 50% 3.5 1.4 1.57 0.39 240 8% 30% 62% 3.1 1.8 1.83 0.48 Data from E. A. Newsholme & A. R. Leach (1983) Biochemistry for the Medical Sciences, John Wiley, NY. pp 370-372. Muscle contraction Components involved in Muscle contraction Role of Ca in Muscle contraction The muscle cell action potential leads to release of calcium into the cytoplasm from the sarcoplasmic reticulum (SR). • • • • • Normally, cytoplasmic calcium level is maintained at a low level by the activity of a calcium pump in the SR membrane. The calcium pump uses the energy from: ATP to ADP and P, to pump calcium into the SR. This process uses energy and creates a concentration gradient (active transport). Thus, calcium levels in the SR are high and there is a gradient between the cytoplasm and the SR. When an muscle action potential enters the proper region of the muscle cell, it triggers the opening of calcium channels in the membrane of the SR. Calcium will flow out of the SR into the cytoplasm by diffusion. Contraction will be triggered. Finally, calcium will be pumped back into the SR by the calcium pump, this will allow relaxation to occur by removing calcium from the cytoplasm, it will also reset the calcium gradient so that the next contraction can be triggered. Energy usage during muscle contraction is due both the ATP use by myosin to energize myosin heads and also due to use of ATP by calcium pumps to restore low levels of intracellular calcium and enable relaxation Interaction between Actin and myosin in the presence of calcium. – In the absence of calcium, interaction between myosin and actin is prevented by the presence of tropomyosin, which covers the myosin binding sites on actin. – When calcium levels rise, calcium binds to troponin. The binding of calcium changes the shape (conformation) of troponin. In turn, troponin interacts with tropomyosin and changes its conformation such that it no longer prevents actin-myosin interaction. – Contraction can occur until calcium is removed from troponin. Structural correlates of muscle function – Muscles that contract and relax quickly (i.e. a those of a sprinter) need large amounts of sarcoplasmic reticulum with many calcium pumps and channels. – Muscles that are very strong (i.e. those of a weight-lifter) need large amounts of myosin and actin. – Muscles that are very fatigue resistant (i.e. those of a marathon runner) need large amounts of mitochondria to supply ATP. – There are overlaps between these classes of muscle. Sprinters also need to produce high levels of force and thus also probably have high levels of actin and myosin. – However, tradeoffs are needed because there is only a limited amount of space in a muscle, and the relative amounts of each component are important. Sliding-filament model of Muscle contraction • • • • • An action potential originating in the CNS, reaches the axon of the motor neuron. The action potential activates voltage gated calcium ion channels on the axon, and calcium rushes in. The calcium causes the neurotransmitter, acetylcholine, within vesicles in the axon to fuse with the plasma membrane, releasing the acetylcholine into the synapse between the axon and the motor end plate specialization in the sarcolemma of the muscle. The acetylcholine diffuses across the synapse and binds to nicotinic receptors on the motor end plate, opening channels in the membrane for sodium and potassium. Sodium rushes in, while potassium trickles out through the sodium-potassium (Na/K) pump located in the sarcolemma However, because sodium is more permeable, the muscle fiber membrane becomes more positively charged, triggering an action potential. • • • • • The action potential spreads through the muscle fibre's network of T tubules, depolarizing the inner portion of the muscle fibre. The depolarization activates voltage-gated calcium channels in the T tubule membrane, which are in close proximity to calcium-release channels in the adjacent SR. * Activated voltage-gated calcium channels physically interact with calcium-release channels to activate them, causing the SR to release calcium. * The calcium binds to the troponin C present on the thin filaments of the myofibrils. The troponin then allosterically modulates the tropomyosin. Normally the tropomyosin sterically obstructs binding sites for myosin on the thin filament; once calcium binds to the troponin C and causes an allosteric change in the troponin protein troponin T allows tropomyosin to move, unblocking the binding sites. • • • Myosin (which has ADP and inorganic phosphate bound to its nucleotide binding pocket and is in a ready state) binds to the newly uncovered binding sites on the thin filament. Myosin is now bound to actin in the strong binding state (Actino-myosin). The release of ADP and inorganic phosphate causes the myosin head to turn, causing a ratchet movement (Actin acts as a cofactor in the release of inorganic phosphate, expediting the release). This will pull the Z-bands towards each other. It also shortens the sarcomere and the Iband. ATP binds myosin, allowing it to release actin and be in the weak binding state. (A lack of ATP makes this step impossible, resulting in rigor mortis.) The myosin then hydrolyzes the ATP and uses the energy to move into the "cocked back" state while releasing ADP and inorganic phosphate. Evidence indicates that each skeletal muscle myosin head moves 10-12 nm each power stroke, however there is also evidence (in vitro) of variations (smaller and larger) that appear specific to the myosin isoform. • • • • Steps * are repeat as long as ATP is available and calcium is present on thin filament. While the above steps are occurring, calcium is actively pumped back into the sarcoplasmic reticulum. When calcium is no longer present on the thin filament, the tropomyosin changes conformation back to its previous state so as to block the binding sites again. The myosin ceases binding to the thin filament, and the contractions cease. • The calcium ions leave the troponin molecule in order to maintain the calcium ion concentration in the sarcoplasm. • The active pumping of calcium ions into the sarcoplasmic reticulum creates a deficiency in the fluid around the myofibrils. • This causes the removal of calcium ions from the troponin. • Thus the tropomyosin-troponin complex again covers the binding sites on the actin fiaments and contraction ceases. Smooth muscle contraction • The interaction of sliding actin and myosin filaments is similar in smooth muscle. There are differences in the proteins involved in contraction in vertebrate smooth muscle compared to cardiac and skeletal muscle. Smooth muscle does not contain troponin, but does contain tropomyosin and other notable proteinscaldesmon and calponin. Contractions in vertebrate smooth muscle are initiated by agents that increase intracellular calcium. This is a process of depolarizing the sarcolemma and extracellular calcium entering through L type calcium channels, and intracellular calcium release predominately from the SR. • Calcium release from the SR is from Ryanodine receptor channels (calcium sparks) by a redox process and Inositol triphosphate receptor channels by the second messenger inositol triphosphate. The intracellular calcium binds with calmodulin which then binds and activates myosin-light chain kinase. The calcium-calmodulin-myosin light chain kinase complex phosphorylates the 20 kilodalton (kd) myosin light chains on amino acid residue-serine 19 to initiate contraction and activate the myosin ATPase. The phosphorylation of caldesmon and calponin by various kinases is believed to play a role in smooth muscle contraction. Space-filling model of the head of muscle myosin. The model is oriented so that the actinbinding surface is located at the lower right-hand corner. Three domains of the myosin heavy chain are colored green, red, and blue, respectively, whereas the two light chains are shown in yellow and purple. ATTACHED - At the start of the cycle a myosin head lacking a bound nucleotide is locked tightly onto an actin filament in a rigor configuration (so named because it is responsible for rigor mortis, the rigidity of death). In an actively contracting muscle this state is very shortlived, being rapidly terminated by the binding of a molecule of ATP. • • RELEASED - A molecule of ATP binds to the large cleft on the "back" of the head and immediately causes a slight change in the conformation of the domains that make up the actin-binding site. This reduces the affinity of the head for actin and allows it to move along the filament. COCKED - The cleft closes around the ATP molecule, triggering a large shape change that causes the head to be displaced along the filament by a distance of about 5 nm. Hydrolysis of ATP occurs, but the ADP and Pi produced remain tightly bound to the protein. • • FORCE-GENERATING - The weak binding of the myosin head to the new site on the actin filament causes release of the inorganic phosphate produced by ATP hydrolysis, concomitantly with the tight binding of the head to actin. This release triggers the power stroke - the forcegenerating change in shape during which the head regains its original conformation. In the course of the power stroke, the head loses its bound ADP, thereby returning to the start of a new cycle. ATTACHED - At the end of the cycle, the myosin head is again locked to the actin filament in a rigor configuration. Note that the head has moved to a new position on the actin filament. Action wave Calcium wave