* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Potential
Circular dichroism wikipedia , lookup
Standard Model wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Elementary particle wikipedia , lookup
Potential energy wikipedia , lookup
Maxwell's equations wikipedia , lookup
Magnetic monopole wikipedia , lookup
Fundamental interaction wikipedia , lookup
Lorentz force wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Cation–pi interaction wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
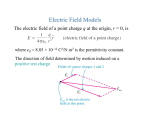
PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Announcements: HW 5 corrected, HW 6 given out, FCQs this Wednesday Electrostatics in Ionic Solutions Last lecture: talked about pH This lecture: Brief review of electrostatics and introduction to idea of screening length Applications of electrostatistics in the cell DNA molecule can be seen as a charged line with a linear charge density. To simplify matters, we can assume the DNA molecule to be an infinitely long straight line. Then the DNA exhibits a cylindrical symmetry. In the cell, the plasma membrane can be seen as a charged plane with an area charge density. The electrostatic field can be computed with Gauss law. Restricting oneself to an infinitely large surface with negligible curvature. Besides its function as a diffusion barrier, the biomembrane acts as a parallel plate capacitor because charges are separated by active processes such as ion pumps. Therefore biomembranes can be modeled as two oppositely charged planes. Charged surfaces trap counterions The surfaces of large proteins, nucleic acids, cell membranes, and many other surfaces relevant to biology, are often charged. The charges are often important for solubulizing the proteins or membranes. Those charged surfaces, when immersed in solution where ions are present, will attract a thin `atmosphere' of opposite-charge counterions. Our goal is to calculate the thickness of this layer, screening length As you might guess, an implication of this is that charged surfaces, and therefore biomolecules, only interact by Coulomb interactions when they are less than a few from one another. They must be a few nm from one another to `feel' one another, and to interact. This leads to perhaps the only thing that is a simplification of molecular biology relative to chemical engineering - in general we can think of the Coulomb interaction as being a short-ranged interaction, or even a `contact' interaction. Screening of Electrostatic Interactions Interactions between charges relevant in biology arw usually reduced in strength or screened - by the presence of many nearby water molecules, ions, and other molecules. TODAY: We have the idea that if we have a bare + charge in solution the – charges ought to move toward it and screen it out. What is the mathematics of this? 1 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Review of electrostatics Monopole E (r) 1 q (in vacuum, unscreened) rˆ 4 o r 2 ε0 = 8.85 × 10−12 F/m or C2/(Nm2) (vacuum permittivity, permittivity of free space) We call this a long-ranged interaction because of its slow power-law decay. In vacuum, the field lines starting out from an isolated charge flow off to infinity without terminating. Charges embedded in a dielectric material interact by a Coulomb interaction which is reduced in strength by a dimensionless factor called the dielectric constant r 1 q r̂ E(r ) (in a material with dielectric constant r ) 4 o r r 2 This reduction in strength of the Coulomb interaction is due to the polarization of the particles of the dielectric medium - either induced or permanent dipoles around a free charge will be oriented so as to terminate some of the field lines coming from the free charge. Note that the Coulomb interaction continues to have its long-ranged character, just with a reduced strength. This is sometimes referred to as dielectric screening of the charge. Dipole + d Define the dipole moment _ p=qd The electric field from a dipole is complicated, but at large distances goes like p/r3 There are two kinds of dipoles 1) Permanent 2) Induced Water has a permanent electric dipole p = 6.2 x 10-30 C m d + H + - O 2 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Other molecules can have an induced dipole moment when placed in an electric field - - +q ++ Separation of charge produces and induced dipole moment Induced dipoles generally have a much smaller dipole moment than molecules with a permanent dipole. Force In electrostatics, the force on a test particle with charge q2 is given by Coulomb’s law F ( r ) q2 E ( r ) q2 q1 rˆ 4 o r r 2 1 Potential and Potential Energy Recall that to find a potential energy we first need to calculate the work. In order to bring two like charges near each other work must be done. The closer the charges are moved together, the more electrical potential energy the charge has. Electric potential from a point charge If we have a particle of charge Q at the origin, and if we move a test particle with charge q from point a to point b we get q b Work = F( x ) dx Q a a b Qq dx 4 o r x 2 a Qq 4 o r 1 1 b a 3 b This work is + because b > a PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Now the work is also the negative of the change in potential energy Work = - (Change in Potential Energy) = - U = - (Ufinal – Uinitial) This suggests that Qq U(x) = 4 o r 1 x The electric potential (written as V(x) or ( x ) ) is Q V(x) = U(x)/q = 4 o r 1 x (goes like 1/distance) The point is that the potential energy is given by U(x) = qV(x) = (charge) (potential) Electric Potential in general b V( x ) E( x ) d s a Also there is the inverse connection E( x) V(x) In E&M classes, we used this to derive Laplace's equation starting from the Maxwell Equation ( x) V ( x) 2 o r , where ρ(x) is the volume charge density. This expression implies that charges are the sources for the electrostatic potential. 4 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Electrostatic screening length We have the idea that if we have a bare + charge in solution the – charges ought to move toward it and screen it out. What is the mathematics of this? We will do a one-dimensional calculation. We have three ideas 1) The concentration of charge being at position x is given by C (x) CoeU( x ) CoezeV ( x ) positive ions C (x) Co e U( x ) Co e zeV ( x ) negative ions Here the charge on the ions is given by ze = (number of electrons added or subtracted) (charge on one electron) 2) The charge density is given by (x) zeC (x) zeC (x) 3) The charge density is related to the potential by Laplace's equation ( x ) V( x ) or 2 In our one-dimensional world this is d 2 V( x ) dx 2 ( x ) or We have to solve these three coupled equations together. Combining the equations, we get d 2 V( x ) dx 2 zeCo zeV ( x ) e e zeV ( x ) o r This equation is not that easy to solve analytically. But it can easily be solved in the limit that the potential V(x) is small compared to temperature. In that case e zeV ( x ) 1 zeV( x) e zeV ( x ) 1 zeV(x) 5 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Our equation becomes d 2 V( x ) dx 2 2(ze) 2 C o o r kT V( x ) The solution is given by potentials of the form V( x) Aex where 2(ze) 2 C o o r kT It is more common to introduce a screening length 1/ So o r kT 2(ze) 2 C o λ is also called the Debye length measuring the amount of screening. It is the distance at which the electric potential due to a point charge in an electrolyte has dropped off by a certain factor. It depends on the ionic concentration of the liquid and it decreases rapidly as the ionic concentration increases. The most general solution will be V(x) Aex / Be x / We can solve for A and B using some kind of boundary conditions. First, often one has a condition V(x = ) = 0 This gives us that B = 0. (Note – in a bounded region this would not be the right BC) 6 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 To figure out the other boundary condition, we need to know something about the source. Suppose the source is a large sheet of charge. E x Charge density The E field from a sheet of charge is given by (Gauss’s Law) E or This will be the value right near the surface as well because the ions can't get this close. But we can also calculate the E field from our expression for V(x). E d d V( x ) Ae x / (A / )e x / dx dx Now setting the E field values at the surface (x=0) equal we get A or So A or And the complete answer is V( x ) x / e or Clearly the most important thing we have learned here is the idea of the screening length. What is it? 7 PES 3950/PHYS 6950 Spendier Spring 2015 Lecture 27 Let's connect it to our idea of pH. Recall [H+] = 10-pH where [H+] is measured in moles/liter We need to change this to a concentration (particles/liter) so multiply by Avagadro's number Co = 6.02 x 1023 [H+] = 6.02 x 1023 (10-pH) Then use o r kT 2(ze) 2 C o With e = 1.602 × 10-19 Coulombs = 8.854 × 10-12 m-3 kg-1 s4 A2 r = 75 (because of the permanent electric dipole) k = 1.38 × 10-23 m2 kg s-2K-1 T = 310 K Z=1 We get pH Screening length (microns) 1 3 5 7 8 9 10 .03 .3 3 30 96 303 959 Of course there could be other ions (Na, Cl) which are not included in the pH measurement. So this represents the largest possible screening length. In typical body concentrations of ions the screening length is a few nm or less. 8