* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2/09 Transpostion of the Great Arteries
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Artificial heart valve wikipedia , lookup
Aortic stenosis wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
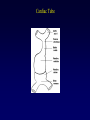
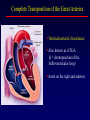
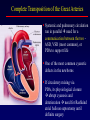
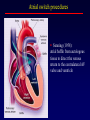
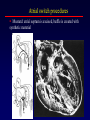
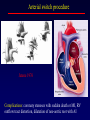
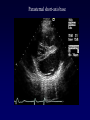
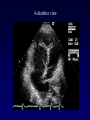
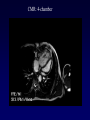
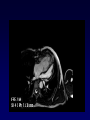
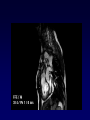
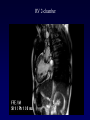
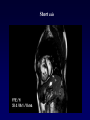
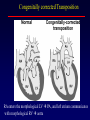
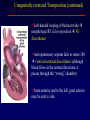
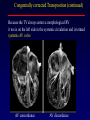
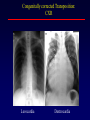
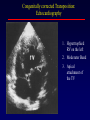
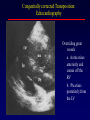
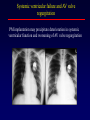
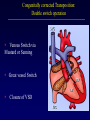
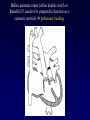
Transposition of the Great Arteries Francesca N. Delling, MD January 11, 2009 Cardiac Tube Complete Transposition of the Great Arteries Ventriculoarterial discordance Also known as d-TGA (d = dextroposition of the bulboventricular loop) Aorta on the right and anterior Complete Transposition of the Great Arteries Systemic and pulmonary circulation run in parallel need for a communication between the two ASD, VSD (most common), or PDA to support life One of the most common cyanotic defects in the newborns If circulatory mixing via PDA, its physiological closure abrupt cyanosis and deterioration need for Rashkind atrial balloon septostomy until definite surgery Atrial switch procedures Senning (1958): atrial baffle from autologous tissue to direct the venous return to the contralateral AV valve and ventricle Atrial switch procedures Mustard: atrial septum is excised, baffle is created with synthetic material Late complications of atrial switch procedures: Arrhythmias Late development of both brady and tachyarrhythmias Sinus node dysfunction common in adults Series of 534 children by Gelatt et al (J Am Coll Cardiol.1997;29:194-201) - NSR in 77% at 5 years, 40% at 20 years - 11% needed PPM - 14% had atrial flutter - 16% had a late death (due to arrhythmias and myocardial failure) Ventricular arrhythmias uncommon in the absence of severe ventricular dysfunction Late complications of atrial switch procedures: Systemic Ventricular dysfunction and TR RV is the systemic ventricle TR secondary to annular dilatation (TVR not indicated) or damage at the time of VSD repair or endocarditis (TVR warranted) Treatment of systemic ventricular dysfunction is challenging: - No convincing data of utility of ACE-inhibitors - Caution with the use of beta-blockers (AVB, bradycardia) - Two-stage repair surgery (pulmonary artery banding to “retrain” the LV, followed by baffle take-down and arterial switch) is extensive and LV failure occurs after pulmonary banding Late complications of atrial switch procedures: Atrial baffle obstruction and leaks Obstruction of SVC (with “SVC syndrome”) more common than IVC (hepatic congestion or cirrhosis). If significant stenosis, may need percutaneous balloon and stenting or even surgery Pulmonary vein stenosis much less common but may cause PHTN Late complications of atrial switch procedures: Pulmonary hypertension Occurs in 7% of those who survive to adulthood Cause unclear (in some cases: PV baffle obstruction) Risk factors: age > 2, shunts at ventricular or great artery level before repair (Newfeld EA et al. Am J Cardiol.1974;34:539-543) Comparison between atrial switch procedures Senning better than Mustard 340 pts (124 Mustard vs. 215 Senning) Heart, 2004; 90:307-313 • Mortality – 82 deaths (24%) » Senning showed trend to better mortality but not statistically significant • Baffle Obstruction – 15% - Mustard – 1% - Senning • Senning patients had better functional status Atrial switch procedures and pregnancy Pregnancy poses a significant volume load that may increase RV dimensions and is sometimes irreversible Poses a definite risk of deterioration of functional class, even if pt is asymptomatic Risk of congenital heart disease in the offspring, in the absence of a family history, is probably < 5% (Clarkson PM et al. J Am Coll Cardiol.1994;24:190-193) Arterial switch procedure Jatene 1976 Complications: coronary stenoses with sudden death or MI, RV outflow tract distortion, dilatation of neo-aortic root with AI Rastelli procedure Used when d-TGA + large subaortic VSD and pulmonary stenosis A patch directs blood from the LV through the VSD to the aorta (1) PV is oversewn and a valved conduit is inserted from the RV to the PA to bypass the pulmonary stenosis (2) Rastelli procedure (continued) Advantages: LV functions as systemic ventricle Disadvantages: - Conduit degeneration and stenosis, necessitating reoperation - Atrial and ventricular arrhythmias with sudden death - Possible RV and LV failure Echo: parasternal long-axis Posterior pulmonary artery and anterior aorta aligned rather than in the usual crossing arrangement Parasternal short-axis Aorta is anterior and on the right Parasternal short-axis base 4-chamber view CMR: 4-chamber LVOT RV 2-chamber Short axis Congenitally corrected Transposition RA enters the morphological LV PA, and left atrium communicates with morphological RV aorta Congenitally corrected Transposition (continued) Left-handed looping of the heart tube morphologic RV in levo-position AV discordance Aortopulmonary septum fails to rotate 180 ventriculoarterial discordance (although blood flows in the normal directions, it passes through the “wrong” chamber) Aorta anterior and to the left, great arteries may be side to side Congenitally corrected Transposition (continued) Because the TV always enters a morphological RV it too is on the left side in the systemic circulation and is termed systemic AV valve AV concordance AV discordance Congenitally corrected Transposition: Associated anomalies VSD (70%): if large (LR flow), pts present in infancy or childhood with congestive heart failure. Reversal of flow R L leads to desaturation Pulmonary stenosis (40%), commonly subvalvular. Associated valvular PS also occurs Abnormalities of the systemic (tricuspid) AV valve (90%) Ebstein’s anomaly: - apical displacement of valve BUT no “saillike” anterior leaflet Congenitally corrected Transposition: Conduction anomalies AV node and His have unusual position Many have dual AV nodes Conduction system is vulnerable to fibrosis 2% per year incidence of CHB Congenitally corrected Transposition: CXR Levocardia Dextrocardia Congenitally corrected Transposition: EKG Ventriculuar excitation begins at the septal endocardium of the right sided LV Congenitally corrected Transposition: Echocardiography 1. Hypertrophied RV on the left 2. Moderator Band 3. Apical attachment of the TV Congenitally corrected Transposition: Echocardiography Overriding great vessels a. Aorta arises anteriorly and comes off the RV b. PA arises posteriorly from the LV Congenitally corrected Transposition: Echocardiography anterior and leftward aorta Congenitally corrected Transposition: Cardiac cath: - hemodynamic assessment of associated anomalies - evaluation of systemic AV valve regurgitation and ventricular function MRI: Defines ventricular function and volumes Systemic ventricular failure and AV valve regurgitation ? Cause Inherent vulnerability to failure - concordant coronary anatomy: morphological RV perfused by single right coronary artery with limitations of perfusion -Acar et al. Heart 1999: 20 pts with L-TGA and ETT MIBI all 20 had perfusion defects at rest, 17 worsened with exercise Controversy exists as to which comes first: AV valve regurgitation or ventricular failure Systemic ventricular failure and AV valve regurgitation PM implantation may precipitate deterioration in systemic ventricular function and worsening of AV valve regurgitation Congenitally corrected Transposition: Surgical repair Two schools of thought: – Classic approach – fix only the defects associated with the condition (VSD, systemic AV valve regurgitation) – Newer approach is to restore the LV as the systemic ventricle (anatomical repair) • Major advantage is drastically reduces risk of TR which is associated with RV failure and late sudden death Congenitally corrected Transposition: Classical repair 118 pt classical repair J Thorac Cardiovasc Surg 117 (1999), pp. 1190–1203 – 15 % operative mortality – 56% underwent reoperation within 20 yrs – 48% survival rate at 20 years – CHF > 70% Congenitally corrected Transposition: Double switch operation Venous Switch via Mustard or Senning Great vessel Switch Closure of VSD Congenitally corrected Transposition And VSD A patch can be inserted to tunnel the LV flow into the aorta, and the morphological RV is connected to the pulmonary artery via conduit “Rastelli technique” Before anatomic repair (either double switch or Rastelli) LV needs to be prepared to function as a systemic ventricle pulmonary banding Congenitally corrected Transposition: Anatomic repair 54 pt restoration of LV – J Thorac CV Surg. 2003;125:1229-41 – Early mortality 5.6 % (3 pts) – Long term mortality: • 1 year – 94% survival • 4 year – 90% survival • 9 year – 90% Survival vs. Classic 60 – 83% (10 years) Congenitally corrected Transposition: Anatomic repair Early mortality of 5% encouraging, but: - 7 pts with Rastelli needed repeat conduit, aortic valve replacement or transplantation. - Balloon angioplasty of baffle obstruction or pulmonary stenosis also needed Need for re-intervention post anatomic repair confirmed by more recent paper by Sharma et al (J Thorac Cardiovasc Surg. 2009 Feb;137) - 68 patients (31 Rastelli/atrial switch; 37 arterial switch/atrial rerouting) - early deaths, late re-operations, late deaths (LV dysfunction, tachyarrhytmias observed in both groups Pregnancy and C-TGA Women with systemic EF <40% or significant systemic AV valve regurgitation should be counseled against pregnancy Series of Connolly et al ( J Am Coll Cardiol.1999;33:1962) 60 pregnancies 22 ♀ resulting in 50 live births (83%) • none of the offspring had congenital heart disease • no pregnancy related deaths • 1 woman with significant AV valve regurgitation • 1 had CHF 45 pregnancies 19 ♀ (Am J. Card. 1999;84:820-24) • 27 live births; 12 miscarriages; 6 elective term • 1 child with a congenital heart defect • Mothers with 6 CV events –3 episodes of CHF –2 Episodes of increasing cyanosis, one CVA Antibiotic prophylaxis Prophylaxis is reasonable in patients with CHD with the highest risk for adverse outcome from infective endocarditis - patients with prosthetic valves - previous endocarditis - unrepaired and palliated CHD - repaired CHD within 6 months after the procedure - repaired CHD with residual defects at or adjacent to the site of a prosthetic patch or prosthetic device that inhibits endothelization ACC/AHA guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol. 2008 Dec 2;52(23):e1-121 References Carole A. Warnes. Transposition of the great arteries. Circulation 2006;114:2699-2709. Yale University School of Medicine. Congenital Heart Disease Echo Atlas. http://www.med.yale.edu/intmed/cardio/chd/ http://pediatriccardiology.uchicago.edu/MP/embryology/embryolo gy.htm Thank you