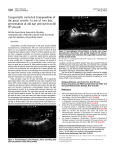
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Clinical Conference
Saturated fat and cardiovascular disease wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Infective endocarditis wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Electrocardiography wikipedia , lookup
Artificial heart valve wikipedia , lookup
Rheumatic fever wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Congenital heart defect wikipedia , lookup
Atrial septal defect wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
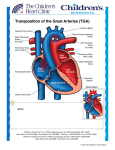
Transposition of the Great Arteries Eric Osborn January 27, 2010 Outline Definitions Embryology Epidemiology Complete transposition (D-TGA) Congenitally corrected transposition (L-TGA) Echocardiography Definitions The key anatomic characteristic of transposition complexes is ventriculoarterial discordance. The aorta arises from the morphological RV The PA arises from the morphological LV Definitions Complete transposition (D-TGA) Atrioventricular concordance Definitions Congenitally corrected transposition (L-TGA) Atrioventricular discordance Embryology 22 days gestation … the primitive straight cardiac tube is formed Embryology 23 days gestation … the straight cardiac tube elongates and bends forming the cardiac loop. Cephalic portion bends ventrally, caudally, and right-ward. Caudal portion moves dorsally, cranially, and left-ward. The rotational motion folding over of the bulboventricular portion bringing the future ventricles side-by-side. Embryology 4th-7th weeks gestation … the heart divides into 4 chambers via formation of swellings (cushions) of tissue that exhibit differential growth. Endocardial cushions divide the AV canal forming the mitral and tricuspid valves. Conotruncal cushions form the outflow tracts, aortic and pulmonary roots. Embryology 5th week gestation … the conotruncal cushions. Right superior truncal cushion grows distally and left-ward. Left inferior truncal cushion grows distally and right-ward. The net effect is a twisting motion. The truncal cushions fuse to form the truncal septum. Additional cushions develop in the conus which grow down and towards each other until they fuse with the truncal septum to form the RVOT and LVOT. Embryology Mechanism of great artery transposition Conotruncal cushion defect Leads to failure of the conotruncal septum to spiral and instead extends straight downward Aorta fuses with the RV and PA with the LV Epidemiology ~0.8% of live births are complicated by a cardiovascular malformation*. >750,000 adult patients with congenital heart disease. Transposition of the great arteries occurs in approximately 1 per 5,000 live births. More common in males Diagnosis possible in utero with fetal echocardiography Transvaginal ultrasound at 13-14 weeks (limited views) Transabdominal ultrasound at 16 weeks *not including bicuspid aortic valve and mitral valve prolapse Complete transposition (D-TGA) Pulmonary and systemic circulations are in parallel Lethal, if no mixing (ASD, PDA, VSD) ¾ are simple with no major associated abnormalities ¼ are complex VSD (16%) Pulmonary/subpulmonary stenosis (9%) Coarctation of the aorta (4%) Complete transposition (D-TGA) Clinical Presentation and Outcomes Larger size and weight at birth Dyspnea and cyanosis Progressive hypoxemia Congestive heart failure Without treatment, the outlook is dismal 30% mortality within the 1st week 90% mortality within the 1st year Complete transposition (D-TGA) Management Prostaglandin E1 to maintain the PDA Atrial septostomy (balloon or surgical) Palliative prior to corrective surgery Repair within the first days to weeks of life 2-4% mortality with 90% 1 year survival Atrial switch Mustard or Senning Arterial switch Rastelli procedure Complete transposition (D-TGA) Atrial switch (Mustard/Senning) Developed in the 1950s Baffle directs venous return to contralateral ventricle Complete transposition (D-TGA) Atrial switch (Mustard/Senning) Disadvantages RV functions as the systemic ventricle Several significant long term complications Congestive heart failure Arrhythmias Baffle leaks and obstruction Pulmonary hypertension Paradoxial embolus Endocarditis Overall survival 75% at 25 years Senning may be better than Mustard [Moons et al, Heart 2004] 340 patients (~⅔ Senning) compared Less obstruction (1 vs. 15%) and better functional class with Senning No significant mortality benefit Complete transposition (D-TGA) Atrial switch (Mustard/Senning) Arrhythmias Palpitations, presyncope, and syncope are not uncommon Both brady and tachyarrythmias frequently seen 50% develop sinus node dysfunction Physical damage during surgery and baffle construction Disruption of blood supply leading to ischemia 20% develop atrial flutter Sensitive to nodal agents due to conduction system disease 11% required pacemakers at 20 years [Gelatt et al, J Am Coll Cardiol 1997] Pacemakers are difficult to place due to distorted anatomy Should be avoided if residual intracardiac communications due to risk of paradoxical embolus and stroke Complete transposition (D-TGA) Atrial switch (Mustard/Senning) Congestive heart failure Most adult patients develop congestive heart failure By 20 years most are NYHA Class I or II RV filling compromised due to defects in baffle construction Baffle leaks (Mustard>Senning) Left-to-right shunts with pulmonary hypertension (7%) Risk of paradoxical embolus and stroke Indications for intervention include >1.5:1 left-to-right shunt or any right-to-left shunt Baffle obstruction (5-15%, Mustard>Senning) SVC>IVC manifesting as SVC syndrome or hepatic congestion/cirrhosis Often undetected due to collateral venous drainage (e.g. azygous vein) 40% develop right ventricular dysfunction 10-40% develop 2+ or greater tricuspid (systemic AV valve) regurgitation Annular dilatation from RV failure Damage from surgery or endocarditis Complete transposition (D-TGA) Suggested Follow-up Complete transposition (D-TGA) Arterial switch Developed in the 1980s Great arteries and coronaries are transected and reanastamosed Complete transposition (D-TGA) Arterial switch Advantages LV is the systemic pump No disruption of atrial conduction (sinus rhythm) Fewer long term complications compared to atrial switch Coronary ostial stenosis Supravalvular pulmonary/aortic stenosis Intervention indicated for RVOT gradient >50 mmHg Neoaortic regurgitation Arrhythmias Follow up with normal LV function and good exercise capacity Complete transposition (D-TGA) Rastelli procedure TGA with VSD and LVOT obstruction Outcomes RV-PA conduit obstruction Exercise intolerance/angina RV failure Intervention for RV-PA gradient >50 mmHg LV-Ao patch obstruction Dyspnea or syncope Complete transposition (D-TGA) RV Failure after Atrial Switch Standard heart failure therapies are unproven The two-stage arterial switch Stage 1 – the PA is banded to ‘re-train’ the LV to handle systemic pressures Stage 2 – the atrial baffles and pulmonary band are taken down and an arterial switch is performed 50% survival at 8 years in early results Appears to be more successful in patients under 12 Congenitally corrected transposition (L-TGA) A rare disorder that may present in adulthood. Associated anomalies (95% of patients) VSD (75%, commonly perimembranous) Pulmonary stenosis (75%, commonly subvalvular) Tricuspid valve anomalies (>75%) Congenital complete heart block (5%) Congenitally corrected transposition (L-TGA) Outcomes Arrhythmias Abnormal AV node and His positions Dual AV nodes 2% per year incidence of complete heart block Susceptible to fibrosis of conduction system Median survival 40 years Mortality from progressive RV failure or arrhythmias Tricuspid regurgitation is major predictor Congenitally corrected transposition (L-TGA) Double Switch Procedure Echocardiography Segmental approach to congenital heart disease 1. 2. Position of the apex Situs of the atria 3. Morphological atria based on anatomic appearance of their appendages 75% concordance with abdominal situs (aorta and IVC positions) Atrioventricular relationship Differentiate the morphological RV from LV: 1. 2. 3. 4. 4. Trabeculated apex Moderator band Septal attachment of the tricuspid valve Lower (apical) insertion of the tricuspid valve Ventriculoarterial relationship Pulmonary artery is distinguished by its early branching pattern Curved contour of the aortic arch with three major branches Echocardiography Complete Transposition with Atrial Switch Hallmark is parallel great arteries (parasternal long axis) Aorta is anterior to PA Echocardiography Complete Transposition with Atrial Switch Systemic hypertrophied RV septum bows into LV May impact TR and enhance subpulmonary stenosis Echocardiography Complete Transposition with Atrial Switch Aortic and pulmonic valves lie in the same plane Aorta is anterior and to the right (parasternal short axis) Echocardiography Congenitally Corrected Transposition Hallmark is reversed offsetting of the AV valves Aorta is anterior and to the left (parasternal short axis) Echocardiography Special Considerations Atrial switch RV function Tricuspid regurgitation Subpulmonary obstruction Baffle leak or obstruction (color Doppler) Arterial switch Normal baffle flow is phasic with peak velocity <1 m/sec Neoaortic valve regurgitation Supraneopulmonary valve stenosis Wall motion abnormalities due to coronary artery ostial stenosis Rastelli procedure LV-Ao tunnel patch obstruction RV-PA conduit degeneration (stenosis/regurgitation) Endocarditis Prophylaxis ACC/AHA 2008 Guidelines state that antibiotic prophylaxis is reasonable to consider for patients at the highest risk of adverse outcomes (Class IIa) Prosthetic valves Prior endocarditis Congenital heart disease Unrepaired cyanotic, including palliative shunts and conduits Completely repaired with prosthetic material or device (6 months) Repaired with defects at or near a prosthetic device Post-cardiac transplant with valvular disease Endocarditis Prophylaxis References Webb et al., Congenital Heart Disease in Braunwald’s Heart Disease, 8th ed., Chapter 61, 1561-1624. Sadler, Cardiovascular System in Langman’s Medical Embryology, 8th ed., Chapter 11, 208-259. Otto, The Adult with Congenital Heart Disease in Clinical Echocardiography, 4th ed., Chapter 17, 418-447. Warnes, Transposition of the Great Arteries, Circulation 2006 114:2699-2709. Love et al., Evaluation and Management of the Adult Patient with Transposition of the Great Arteries Follow Atrial-level (Senning or Mustard) Repair, Nature Clinical Practice Cardiovasc Med 2008 5:454-67. Verhuegt et al., Long-term Prognosis of Congenital Heart Defects: A Systematic Review, Int J Cardiol 2008 131:25-32. Skinner et al., Transposition of the Great Arteries: from Fetus to Adult, Heart 2008 94:1227-35 ACC/AHA Guidelines for the Management of Adults with Congenital Heart Disease, J Am Coll Cardiol 2008 52:e1-121.